Understanding the Electronic Structure and Stability of BnXn0/2– (n = 4, 6; X = H, F, Cl, Br, I, At, Ts) Clusters†
Ruo-Ya Wang
Henan Key Laboratory of Boron Chemistry and Advanced Energy Materials, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorJing-Xuan Zhang
Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorXue-Lian Jiang
Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorNana Ma
Henan Key Laboratory of Boron Chemistry and Advanced Energy Materials, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorXuenian Chen
Henan Key Laboratory of Boron Chemistry and Advanced Energy Materials, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Green Catalysis Center and College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
Search for more papers by this authorCorresponding Author
Cong-Qiao Xu
Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
E-mail: [email protected]Search for more papers by this authorJun Li
Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Department of Chemistry and Key Laboratory of Organic Optoelectronics & Molecular Engineering of Ministry of Education, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorRuo-Ya Wang
Henan Key Laboratory of Boron Chemistry and Advanced Energy Materials, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorJing-Xuan Zhang
Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorXue-Lian Jiang
Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorNana Ma
Henan Key Laboratory of Boron Chemistry and Advanced Energy Materials, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorXuenian Chen
Henan Key Laboratory of Boron Chemistry and Advanced Energy Materials, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Green Catalysis Center and College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
Search for more papers by this authorCorresponding Author
Cong-Qiao Xu
Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
E-mail: [email protected]Search for more papers by this authorJun Li
Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Department of Chemistry and Key Laboratory of Organic Optoelectronics & Molecular Engineering of Ministry of Education, Tsinghua University, Beijing, 100084 China
Search for more papers by this author†Dedicated to Department of Chemistry, SUSTech, on the Occasion of Her 10th Anniversary.
Main observation and conclusion
Borane clusters and their derivatives have attracted extensive attention in inorganic chemistry due to their fascinating multi-center bonding patterns and physicochemical properties. Here we report a systematic theoretical investigation on the geometry, electronic structure and chemical bonding of BnXn0/2- (n = 4, 6; X = H, F, Cl, Br, I, At, Ts) clusters, attempting to explore their bonding features, exceptional stability and the ligand effect. We find that the electronic structure and stability of BnXn0/2- clusters can be tuned by the size of the boron cage and electronegativity of the ligand. Fragment orbital energy matching and orbital overlap are of great importance to the covalency of the cluster. In addition to the ionic electrostatic interaction that dominates the bonding interaction and decreases as the ligand becomes heavier, multi-center bonding covalency determined by the orbital interaction increases accordingly, attributing to the reducing electronegativity. The σ-donations from the ligand to the boron cage as well as multi-center two-electron (nc-2e) bonding contribute to the σ aromaticity and superb stability. This work reveals the foremost factors that determine the structure and stability of boron clusters, and provides insights into the nature of chemical bonding for species with boron cages and even bulk boron.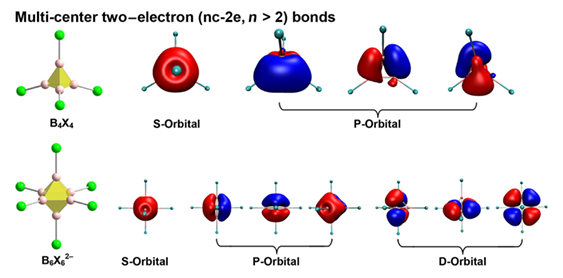
Supporting Information
| Filename | Description |
|---|---|
| cjoc202100072-sup-0001-Supinfo.pdfPDF document, 1.5 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Jian, T.; Chen, X.; Li, S. D.; Boldyrev, A. I.; Li, J.; Wang, L. S. Probing the structures and bonding of size-selected boron and doped-boron clusters. Chem. Soc. Rev. 2019, 48, 3550–3591.
- 2 Li, W. L.; Chen, X.; Jian, T.; Chen, T.-T.; Li, J.; Wang, L. S. From planar boron clusters to borophenes and borospherenes. Nat. Rev. Chem. 2017, 1, 0071.
- 3 Jiang, X.-T.; Hu, X.-G.; Cai, Y.-M.; Li, Q.-S. A further study on closo boron hydrides B16H162− (D2) and B16H16 (Td) using ab initio molecular orbital theory. Chin. J. Chem. 1997, 15, 102–106.
- 4 Wang, H. A density functional investigation of fluorinated B12N12 Clusters. Chin. J. Chem. 2010, 28, 1897–1901.
- 5
Stock, A. Hydrides of boron and silicon. J. Phys. Chem. 1934, 38, 714–715.
10.1021/j150356a019 Google Scholar
- 6 Lipscomb, W. N. Boron Hydrides, W. A. Benjamin, Inc., New York, 1963.
- 7
Wade, K. The structural significance of the number of skeletal bonding electron-pairs in carboranes, the higher boranes and borane anions, and various transition-metal carbonyl cluster compounds. J. Chem. Soc. D 1971, 792–793.
10.1039/c29710000792 Google Scholar
- 8 Stone, A. J. A new approach to bonding in transition metal clusters. Mol. Phys. 1980, 41, 1339–1354.
- 9 Stone, A. J. New approach to bonding in transition-metal clusters and related compounds. Inorg. Chem. 1981, 20, 563–571.
- 10 Mingos, D. M. P. Polyhedral skeletal electron pair approach. Acc. Chem. Res. 1984, 17, 311–319.
- 11 Wade, K. Structural and bonding patterns in cluster chemistry. Adv. Inorg. Chem. Radiochem. 1976, 18, 1–66.
- 12 Williams, E. R. The polyborane, carborane, carbocation continuum: architectural patterns. Chem. Rev. 1992, 92, 177–207.
- 13 Rudolph, R. W. Boranes and heteroboranes: a paradigm for the electron requirements of clusters? Acc. Chem. Res. 1976, 9, 446–452.
- 14 Eberhardt, W. H.; Crawford, B Jr.; Lipscomb, W. N. The valence structure of the boron hydrides. J. Chem. Phys. 1954, 22, 989–1001.
- 15 Longuet-Higgins, H. C.; Roberts, M. D. V.; Randall, J. T. The electronic structure of the borides MB6. Proc. R. Soc. A 1954, 224, 336–347.
- 16 Longuet-Higgins, H. C.; Roberts, M. D. V.; Emeleus, H. J. The electronic structure of an icosahedron of boron atoms. Proc. R. Soc. A 1955, 230, 110–119.
- 17 Schaeffer, R.; Johnson, Q.; Smith, G. S. The crystal and molecular structure of tetramethylammonium hexahydrohexaborate. Inorg. Chem. 1965, 4, 917–918.
- 18 Zint, N.; Dreuw, A.; Cederbaum, L. S. Gas-phase stability of derivatives of the closo-hexaborate dianion B6H62-. J. Am. Chem. Soc. 2002, 124, 4910–4917.
- 19 Schmitt, K.; Stückl, C.; Ripplinger, H.; Albert, B. Crystal and electronic structure of BaB6 in comparison with CaB6 and molecular [B6H6]2-. Solid State Sci. 2001, 3, 321–327.
- 20 Aprà, E.; Warneke, J.; Xantheas, S. S.; Wang, X.-B. A benchmark photoelectron spectroscopic and theoretical study of the electronic stability of [B12H12]2−. J. Chem. Phys. 2019, 150, 164306.
- 21 Pitochelli, A. R.; Hawthorne, F. M. The isolation of the icosahedral B12H12–2 ion. J. Am. Chem. Soc. 1960, 82, 3228–3229.
- 22 King, R. B.; Rouvray, D. H. Chemical applications of group theory and topology. 7. A graph-theoretical interpretation of the bonding topology in polyhedral boranes, carboranes, and metal clusters. J. Am. Chem. Soc. 1977, 99, 7834–7840.
- 23 Mebs, S.; Kalinowski, R.; Grabowsky, S.; Förster, D.; Kickbusch, R.; Justus, E.; Morgenroth, W.; Paulmann, C.; Luger, P.; Gabel, D.; Lentz, D. Real-space indicators for chemical bonding. Experimental and theoretical electron density studies of four deltahedral boranes. Inorg. Chem. 2011, 50, 90–103.
- 24 Warneke, J.; Hou, G.-L.; Aprà, E.; Jenne, C.; Yang, Z.; Qin, Z.; Kowalski, K.; Wang, X.-B.; Xantheas, S. S. Electronic structure and stability of [B12X12]2− (X = F–At): A combined photoelectron spectroscopic and theoretical study. J. Am. Chem. Soc. 2017, 139, 14749–14756.
- 25 Warneke, J.; Konieczka, S. Z.; Hou, G.-L.; Aprà, E.; Kerpen, C.; Keppner, F.; Schäfer, T. C.; Deckert, M.; Yang, Z.; Bylaska, E. J.; Johnson, G. E.; Laskin, J.; Xantheas, S. S.; Wang, X.-B.; Finze, M. Properties of perhalogenated {closo-B10} and {closo-B11} multiply charged anions and a critical comparison with {closo-B12} in the gas and the condensed phase. Phys. Chem. Chem. Phys. 2019, 21, 5903–5915.
- 26 Mayer, M.; van Lessen, V.; Rohdenburg, M.; Hou, G.-L.; Yang, Z.; Exner, R. M.; Aprà, E.; Azov, V. A.; Grabowsky, S.; Xantheas, S. S.; Asmis, K. R.; Wang, X.-B.; Jenne, C.; Warneke, J. Rational design of an argon-binding superelectrophilic anion. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 8167–8172.
- 27 Asmis, K. R.; Beele, B. B.; Jenne, C.; Kawa, S.; Knorke, H.; Nierstenhöfer, M. C.; Wang, X.-B.; Warneke, J.; Warneke, Z.; Yuan, Q. Synthesis, Electronic properties and reactivity of [B12X11(NO2)]2− (X=F–I) dianions. Chem. - Eur. J. 2020, 26, 14594–14601.
- 28 Swanton, D. J.; Ahlrichs, R. Electronic structures of the boron cage molecules B4H4, B4Cl4 and B4F4. Theor. Chem. Acc. 1989, 75, 163–172.
- 29 Olson, J. K.; Boldyrev, A. I. Ab initio search for global minimum structures of neutral and anionic B4H4 clusters. Chem. Phys. 2011, 379, 1–5.
- 30
Atoji, M.; Lipscomb, W. N. The crystal and molecular structure of B4Cl4. Acta Cryst. 2010, 6, 547–550.
10.1107/S0365110X53001472 Google Scholar
- 31 Urry, G.; Wartik, T.; Schlesinger, H. I. A New Sub-Chloride of Boron, B4Cl4. J. Am. Chem. Soc. 1952, 74, 5809–5809.
- 32 Urry, G.; Garrett, A. G.; Schlesinger, H. I. The chemistry of the boron subhalides. I. Some properties of tetraboron tetrachloride, B4Cl4. Inorg. Chem. 1963, 2, 396–400.
- 33 Davan, T.; Morrison, J. A. Tetrakis(t-butyl)tetraborane(4), Bu4tB4; synthesis of the first peralkyl derivative of a 2N framework electron count deltahedral borane. J. Chem. Soc., Chem. Commun. 1981, 250–251.
- 34 Ahmed, L.; Castillo, J.; Morrison, J. A. Chemistry of tetraboron tetrachloride. Synthesis and characterization of tetraboron tetrabromide (B4Br4) and observation of B4BrCl3, B4Br2Cl2, and B4Br3Cl. Inorg. Chem. 1992, 31, 1858–1860.
- 35 Duffey, G. H. The structure of B4Cl4. J. Chem. Phys. 1953, 21, 761–761.
- 36 Mach, P.; Hubač, I.; Mavridis, A. Ab initio structural study of the B4H4 molecule. Asymmetric structure for a ‘symmetric’ system. Chem. Phys. Lett. 1994, 226, 469–474.
- 37 Klanberg, F.; Muetterties, E. L. Chemistry of boranes. XXVII. New polyhedral borane anions, B9H92- and B11H112-. Inorg. Chem. 1966, 5, 1955–1960.
- 38 Preetz, W.; Fritze, J. Darstellung, 11B-NMR- und Schwingungsspektren der oktaedrischen closo-Boratanionen B6X62-; X = H, Cl, Br, I. Z. Naturforsch. B 1984, 39, 1472.
- 39 Rohdenburg, M.; Yang, Z.; Su, P.; Bernhardt, E.; Yuan, Q.; Apra, E.; Grabowsky, S.; Laskin, J.; Jenne, C.; Wang, X.-B.; Warneke, J. Properties of gaseous closo-[B6X6]2− dianions (X = Cl, Br, I). Phys. Chem. Chem. Phys. 2020, 22, 17713–17724.
- 40 Jemmis, E. D.; Prasad, D. L. V. K. Icosahedral B12, macropolyhedral boranes, β-rhombohedral boron and boron-rich solids. J. Solid State Chem. 2006, 179, 2768–2774.
- 41 Jemmis, E. D.; Balakrishnarajan, M. M. The ubiquitous icosahedral B12 in boron chemistry. Bull. Mater. Sci. 1999, 22, 863–867.
- 42 Mayer, I. Charge, bond order and valence in the ab initio SCF theory. Chem. Phys. Lett. 1983, 97, 270–274.
- 43 Reed, A. E.; Weinstock, R. B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–730.
- 44 Tang, W.; Sanville, E.; Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys.: Condens. Matter 2009, 21, 084204.
- 45 Pyykkö, P. Strong closed-shell interactions in inorganic chemistry. Chem. Rev. 1997, 97, 597–636.
- 46 Lin, C.-S.; Li, J.; Liu, C.-W. Theoretical study on tetranuclear boron clusters: B4X4 (X = H, F, Cl, Br, I). Chin. J. Chem. 1994, 12, 305–313.
- 47 Del Bene, J. E.; Alkorta, I.; Elguero, J. B4H4 and B4(CH3)4 as unique electron donors in hydrogen-bonded and halogen-bonded complexes. J. Phys. Chem. A 2016, 120, 5745–5751.
- 48 Shen, Y.-F.; Xu, C.; Cheng, L.-J. Deciphering chemical bonding in BnHn2− (n = 2–17): flexible multicenter bonding. RSC Adv. 2017, 7, 36755–36764.
- 49 Wang, Z.-L.; Hu, H.-S.; von Szentpály, L.; Stoll, H.; Fritzsche, S.; Pyykkö, P.; Schwarz, W. H. E.; Li, J. Understanding the uniqueness of 2p elements in periodic tables. Chem. - Eur. J. 2020, 26, 15558–15564.
- 50 te Velde, G.; Bickelhaupt, F. M.; Baerends, E. J.; Fonseca Guerra, C.; van Gisbergen, S. J. A.; Snijders, J. G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967.
- 51 van Lenthe, E.; Baerends, E. J.; Snijders, J. G. Relativistic regular two-component hamiltonians. J. Chem. Phys. 1993, 99, 4597–4610.
- 52 Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868.
- 53 Lenthe, E.; Baerends, E. Optimized Slater-type basis sets for the elements 1–118. J. Comput. Chem. 2003, 24, 1142–1156.
- 54 Mitoraj, M. P.; Michalak, A.; Ziegler, T. A combined charge and energy decomposition scheme for bond analysis. J. Chem. Theory Comput. 2009, 5, 962–975.
- 55 Foster, J. P.; Weinhold, F. Natural hybrid orbitals. J. Am. Chem. Soc. 1980, 102, 7211–7218.
- 56 Reed, A. E.; Curtiss, L. A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926.
- 57 Elser, V.; Haddon, R. C. Icosahedral C60: an aromatic molecule with a vanishingly small ring current magnetic susceptibility. Nature 1987, 325, 792–794.
- 58 Schleyer, P. v. R.; Maerker, C.; Dransfeld, A.; Jiao, H.; van Eikema Hommes, N. J. R. Nucleus-independent chemical shifts: A simple and efficient aromafcity probe. J. Am. Chem. Soc. 1996, 118, 6317–6318.
- 59 Chen, Z.; Wannere, C. S.; Corminboeuf, C.; Puchta, R.; Schleyer, P. v. R. Nucleus-independent chemical shifts (NICS) as an aromaticity criterion. Chem. Rev. 2005, 105, 3842–3888.
- 60 Zubarev, D. Y.; Boldyrev, A. I. Developing paradigms of chemical bonding: adaptive natural density partitioning. Phys. Chem. Chem. Phys. 2008, 10, 5207–5217.
- 61 Lu, T.; Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592.
- 62 Pritchard, B. P.; Altarawy, D.; Didier, B. T.; Gibson, T. D.; Windus, T. L. New basis set exchange: An open, up-to-date resource for the molecular sciences community. J. Chem. Inf. Model. 2019, 59, 4814–4820.
- 63Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A. V.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery, J. A. Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M. J.; Heyd, J. J.; Brothers, E. N.; Kudin, K. N.; Staroverov, V. N.; Keith, T. A.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A. P.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J. Gaussian 16,, Revision A.03, Gaussian, Inc., Wallingford CT, 2016.




