One Stone Two Birds—Enantioselective Bimetallic Catalysis for α-Amino Acid Derivatives with an Allene Unit
Junzhe Xiao
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorHaibo Xu
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorXiaohong Huo
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering and School of Pharmacy, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorWanbin Zhang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering and School of Pharmacy, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorCorresponding Author
Shengming Ma
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Research Centre for Molecular Recognition and Synthesis, Department of Chemistry, Fudan University, 220 Handan Lu, Shanghai, 200433 China
E-mail: [email protected]Search for more papers by this authorJunzhe Xiao
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorHaibo Xu
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorXiaohong Huo
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering and School of Pharmacy, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorWanbin Zhang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering and School of Pharmacy, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorCorresponding Author
Shengming Ma
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Research Centre for Molecular Recognition and Synthesis, Department of Chemistry, Fudan University, 220 Handan Lu, Shanghai, 200433 China
E-mail: [email protected]Search for more papers by this authorMain observation and conclusion
A highly enantioselective 2,3-allenylation of acyclic and cyclic α-imino carboxylates via a synergistic bimetallic Pd/Cu catalysis with the same commercially available (R,Rp)-iPr-FOXAP (also as Phosferrox, (R,R)-[2-(4’-i-propyloxazolin-2’-yl)ferrocenyl]diphenyl phosphine) ligand for both metals affording optically active 2,3-butadienyl α-amino acid derivatives in high to excellent yields with excellent enantioselectivities has been developed. The synthetic versatility of this reaction has been demonstrated by gram-scale synthesis, a catalytic enantioselective synthesis of naturally occurring (S)-2-amino-4,5-hexadienoic acid A, and conversions to several useful chemicals, such as optically active α-amino acids, β-amino alcohols, potential chiral oxazoline ligands bearing an allenic moiety, and bicyclic ketone compounds. A mechanism involving the roles of two metals and the single chiral ligand has been extensively studied based on the isolation of key palladium pre-catalyst and control experiments.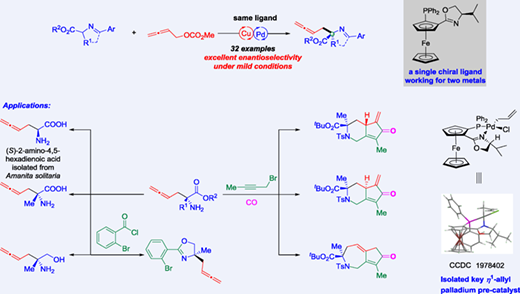
Supporting Information
| Filename | Description |
|---|---|
| cjoc202100002-sup-0001-Supinfo.pdfPDF document, 18.8 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Ager, D. J. Amino Acids, Peptides and Proteins in Organic Chemistry, Ed.: Hughes, A. B., Wiley-VCH, Weinheim, 2009, Vol. 1, pp. 495–526; (b) G. C. Barret; Meienhofer, J. Chemistry and Biochemistry of the Amino Acids, Ed.: Barret, G. C., Chapman and Hall, London & New York, 1985, pp. 246–375.
- 2For reviews, see: (a) Taggi, A. E.; Hafez, A. M.; Lectka, T. α-Imino Esters: Versatile Substrates for the Catalytic, Asymmetric Synthesis of α- and β-Amino Acids and β-Lactams. Acc. Chem. Res. 2003, 36, 10–19; (b) Nájera, C.; Sansano, J. M. Catalytic Asymmetric Synthesis of α-Amino Acids. Chem. Rev. 2007, 107, 4584–4671; (c) Weiner, B.; Szymański, W.; Janssen, D. B.; Minnaard, A. J.; Feringa, B. L. Recent Advances in the Catalytic Asymmetric Synthesis of β-Amino Acids. Chem. Soc. Rev. 2010, 39, 1656–1691.
- 3 Rivera-Fuentes, P.; Diederich, F. Allenes in Molecular Materials. Angew. Chem. Int. Ed. 2012, 51, 2818–2828.
- 4 Hoffmann-Röder, A.; Krause, N. Synthesis and Properties of Allenic Natural Products and Pharmaceuticals. Angew. Chem. Int. Ed. 2004, 43, 1196–1216.
- 5For reviews on chemical transformation of allenes, see: (a) Zimmer, R.; Dinesh, C. U.; Nandanan, E.; Khan, F. A. Palladium-Catalyzed Reactions of Allenes. Chem. Rev. 2000, 100, 3067–3125; (b) Bates, R. W.; Satcharoen, V. Nucleophilic Transition Metal Based Cyclization of Allenes. Chem. Soc. Rev. 2002, 31, 12–21; (c) Sydnes, L. K. Allenes from Cyclopropanes and Their Use in Organic Synthesis-Recent Developments. Chem. Rev. 2003, 103, 1133–1150; (d) Wei, L.-L.; Xiong, H.; Hsung, R. P. The Emergence of Allenamides in Organic Synthesis. Acc. Chem. Res. 2003, 36, 773–782; (e) Ma, S. Some Typical Advances in the Synthetic Applications of Allenes. Chem. Rev. 2005, 105, 2829–2872; (f) Ma, S. Palladium-Catalyzed Two- or Three-Component Cyclization of Functionalized Allenes. Top. Organomet. Chem. 2005, 14, 183–210; (g) Ma, S. Electrophilic Addition and Cyclization Reactions of Allenes. Acc. Chem. Res. 2009, 42, 1679–1688; (h) Alcaide, B.; Almendros, P.; Aragoncillo, C. Exploiting [2+2] Cycloaddition Chemistry: Achievements with Allenes. Chem. Soc. Rev. 2010, 39, 783–816; (i) Yu, S.; Ma, S. Allenes in Catalytic Asymmetric Synthesis and Natural Product Syntheses. Angew. Chem. Int. Ed. 2012, 51, 3074–3112; (j) Lu, T.; Lu, Z.; Ma, Z.-X.; Zhang, Y.; Hsung, R. P. Allenamides: A Powerful and Versatile Building Block in Organic Synthesis. Chem. Rev. 2013, 113, 4862–4904; (k) Ye, J.; Ma, S. Palladium-Catalyzed Cyclization Reactions of Allenes in the Presence of Unsaturated Carbon-Carbon Bonds. Acc. Chem. Res. 2014, 47, 989–1000; (l) Alcaide, B.; Almendros, P. Gold- Catalyzed Cyclization Reactions of Allenol and Alkynol Derivatives. Acc. Chem. Res. 2014, 47, 939–952; (m) Yang, B.; Qiu, Y.; Bäckvall, J.-E. Control of Selectivity in Palladium(II)-Catalyzed Oxidative Transformations of Allenes. Acc. Chem. Res. 2018, 51, 1520–1531; (n) Liu, L.; Ward, R. M.; Schomaker, J. M. Mechanistic Aspects and Synthetic Applications of Radical Additions to Allenes. Chem. Rev. 2019, 119, 12422–12490; (o) Liu, Y.; Bandini, M. Nickel Catalyzed Functionalization of Allenes. Chin. J. Chem. 2019, 37, 431–441; (p) Fu, L.; Greßies, S.; Chen, P.; Liu, G. Recent Advances and Perspectives in Transition Metal-Catalyzed 1,4-Functionalizations of Unactivated 1,3-Enynes for the Synthesis of Allenes. Chin. J. Chem. 2020, 38, 91–100.
- 6(a) Chilton, W. S.; Tsou, G.; Kirk, L.; Benedict, R. G. A Naturally-Occurring Allenio Amino Acid. Tetrahedron Lett. 1968, 9, 6283–6284;
10.1016/S0040-4039(00)75453-0 Google Scholar(b) Baldwin, J. E.; Adlington, R. M.; Basak, A. Allene Transfer Reactions. A New Synthesis of Terminal Allenes. J. Chem. Soc., Chem. Commun. 1984, 1284–1285; (c) Hatanaka, S.-I.; Niimura, Y.; Takishima, K.; Sugiyama, J. (2R)-2-Amino-6-hydroxy-4-hexynoic Acid, and Related Amino Acids in the Fruiting Bodies of Amanita miculifera. Phytochemistry 1998, 49, 573–578.
- 7 Lachance, B.; Salvador, R. L.; Simon, D. Z. Synthesis and Evaluation of Potential Glutamine Antagonists. Eur. J. Med. Chem. 1987, 22, 179–186.
- 8 Casara, P.; Jund, K.; Bey, P. General Synthetic Access to α-Allenyl Amines and α-Allenyl-α-Aminoacids as Potential Enzyme Activated Irreversible Inhibitors of PLP Dependent Enzymes. Tetrahedron Lett. 1984, 25, 1891–1894.
- 9 Castelhano, A. L.; Pliura, D. H.; Taylor, G. J.; Hsieh, K. C.; Krantz, A. Allenic Suicide Substrates. New Inhibitors of Vitamin B6 Linked Decarboxylases. J. Am. Chem. Soc. 1984, 106, 2734–2735.
- 10For seminal reports on Pd-catalyzed asymmetric allenylation with malonates generating axial and/or central chirality, see: (a) Imada, Y.; Ueno, K.; Kutsuwa, K.; Murahashi, S.-I. Palladium-Catalyzed Asymmetric Alkylation of 2,3-Alkadienyl Phosphates. Synthesis of Optically Active 2-(2,3-Alkadienyl)malonates. Chem. Lett. 2002, 31, 140–141; (b) Trost, B. M.; Fandrick, D. R.; Dinh, D. C. Dynamic Kinetic Asymmetric Allylic Alkylations of Allenes. J. Am. Chem. Soc. 2005, 127, 14186–14187; (c) Li, Q.; Fu, C.; Ma, S. Catalytic Asymmetric Allenylation of Malonates with the Generation of Central Chirality. Angew. Chem. Int. Ed. 2012, 51, 11783–11786; (d) Dai, J.; Duan, X.; Zhou, J.; Fu, C.; Ma, S. Catalytic Enantioselective Simultaneous Control of Axial Chirality and Central Chirality in Allenes. Chin. J. Chem. 2018, 36, 387–391; (e) Song, S.; Zhou, J.; Fu, C.; Ma, S. Catalytic Enantioselective Construction of Axial Chirality in 1,3-Disubstituted Allenes. Nat. Commun. 2019, 10, 507; (f) Song, S.; Ma, S. Highly Selective Nucleophilic 4-Aryl-2,3-allenylation of Malonates. Chin. J. Chem. 2020, 38, 1233–1238. For Pd-catalyzed asymmetric allenylation with α-hydroxyketones, see: (g) Trost, B. M.; Schultz, J. E.; Chang, T.; Maduabum, M. R. Chemo-, Regio-, Diastereo-, and Enantioselective Palladium Allylic Alkylation of 1,3-Dioxaboroles as Synthetic Equivalents of α-Hydroxyketones. J. Am. Chem. Soc. 2019, 141, 9521–9526. For Pd-catalyzed asymmetric allenylation with amines, see: (h) Wan, B.; Ma, S. Enantioselective Decarboxylative Amination: Synthesis of Axially Chiral Allenyl Amines. Angew. Chem. Int. Ed. 2013, 52, 441–445; (i) Li, Q.; Fu, C.; Ma, S. Palladium-Catalyzed Asymmetric Amination of Allenyl Phosphates: Enantioselective Synthesis of Allenes with an Additional Unsaturated Unit. Angew. Chem. Int. Ed. 2014, 53, 6511–6514.
- 11For examples of α-imino carboxylates in asymmetric allylic alkylation reaction, see: (a) Huo, X.; He, R.; Fu, J.; Zhang, J.; Yang, G.; Zhang, W. Stereoselective and Site-Specific Allylic Alkylation of Amino Acids and Small Peptides via a Pd/Cu Dual Catalysis. J. Am. Chem. Soc. 2017, 139, 9819–9822; (b) Wei, L.; Xu, S.-M.; Zhu, Q.; Che, C.; Wang, C.-J. Synergistic Cu/Pd Catalysis for Enantioselective Allylic Alkylation of Aldimine Esters: Access to α,α-Disubstituted α-Amino Acids. Angew. Chem. Int. Ed. 2017, 56, 12312–12316; (c) Wei, L.; Zhu, Q.; Xu, S.-M.; Chang, X.; Wang, C.-J. Stereodivergent Synthesis of α,α-Disubstituted α-Amino Acids via Synergistic Cu/Ir Catalysis. J. Am. Chem. Soc. 2018, 140, 1508–1513; (d) Huo, X.; Zhang, J.; Fu, J.; He, R.; Zhang, W. Ir/Cu Dual Catalysis: Enantio- and Diastereodivergent Access to α,α-Disubstituted α-Amino Acids Bearing Vicinal Stereocenters. J. Am. Chem. Soc. 2018, 140, 2080–2084; (e) Wei, L.; Xiao, L.; Wang, C.-J. Synergistic Cu/Pd Catalysis for Enantioselective Allylation of Ketimine Esters: The Direct Synthesis of α-Substituted α-Amino Acids and 2H-Pyrrols. Adv. Synth. Catal. 2018, 360, 4715–4719; (f) Huo, X.; Fu, J.; He, X.; Chen, J.; Xie, F.; Zhang, W. Pd/Cu Dual Catalysis: Highly Enantioselective Access to α-Substituted α-Amino Acids and α-Amino Amides. Chem. Commun. 2018, 54, 599–602; (g) Liu, P.; Huo, X.; Li, B.; He, R.; Zhang, J.; Wang, T.; Xie, F.; Zhang, W. Stereoselective Allylic Alkylation of 1-Pyrroline-5-carboxylic Esters via a Pd/Cu Dual Catalysis. Org. Lett. 2018, 20, 6564–6568; (h) He, R.; Huo, X.; Zhao, L.; Wang, F.; Jiang, L.; Liao, J.; Zhang, W. Stereodivergent Pd/Cu Catalysis for the Dynamic Kinetic Asymmetric Transformation of Racemic Unsymmetrical 1,3-Disubstituted Allyl Acetates. J. Am. Chem. Soc. 2020, 142, 8097–8103. For DFT calculations on the structure of Cu-metallated azomethine ylide: (i) Yan, X.-X.; Peng, Q.; Zhang, Y.; Zhang, K.; Hong, W.; Hou, X.-L.; Wu, Y.-D. A Highly Enantio- and Diastereoselective Cu-Catalyzed 1,3-Dipolar Cycloaddition of Azomethine Ylides with Nitroalkenes. Angew. Chem. Int. Ed. 2006, 45, 1979–1983.
- 12 Liu, H.-C.; Hu, Y.-Z.; Wang, Z.-F.; Tao, H.-Y.; Wang, C.-J. Synergistic Cu/Pd-Catalyzed Asymmetric Allenylic Alkylation of Azomethine Ylides for the Construction of α-Allene-Substituted Nonproteinogenic α-Amino Acids. Chem.-Eur. J. 2019, 25, 8681–8685.
- 13For selected reviews on bimetallic catalysis, see: (a) van den Beuken, E. K.; Feringa, B. L. Bimetallic Catalysis by Late Transition Metal Complexes. Tetrahedron 1998, 54, 12985–13011; (b) Lee, J. M.; Na, Y.; Han, H.; Chang, S. Cooperative Multi-Catalyst Systems for One-Pot Organic Transformations. Chem. Soc. Rev. 2004, 33, 302–312; (c) Park, J.; Hong, S. Cooperative Bimetallic Catalysis in Asymmetric Transformations. Chem. Soc. Rev. 2012, 41, 6931–6943; (d) Fu, J.; Huo, X.; Li, B.; Zhang, W. Cooperative Bimetallic Catalysis in Asymmetric Allylic Substitution. Org. Biomol. Chem. 2017, 15, 9747–9759; (e) Wu, Y.; Huo, X.; Zhang, W. Synergistic Pd/Cu Catalysis in Organic Synthesis. Chem.-Eur. J. 2020, 26, 4895–4916.
- 14For selected examples on racemic bimetallic catalysis, see: (a) Semba, K.; Nakao, Y. Arylboration of Alkenes by Cooperative Palladium/ Copper Catalysis. J. Am. Chem. Soc. 2014, 136, 7567–7570; (b) Chen, Z.-S.; Huang, L.-Z.; Jeon, H. J.; Xuan, Z.; Lee, S.-G. Cooperative Pd(0)/Rh(II) Dual Catalysis: Interceptive Capturing of π-Allyl Pd(II) Complexes with α-Imino Rh(II) Carbenoids. ACS Catal. 2016, 6, 4914–4919; (c) Lee, J. T. D.; Zhao, Y. Access to Acyclic Z-Enediynes by Alkyne Trimerization: Cooperative Bimetallic Catalysis Using Air as the Oxidant. Angew. Chem. Int. Ed. 2016, 55, 13872–13876; (d) Chen, Z.-S.; Huang, X.-Y.; Chen, L.-H.; Gao, J.-M.; Ji, K. Rh(II)/Pd(0) Dual Catalysis: Regiodivergent Transformations of Alkylic Oxonium Ylides. ACS Catal. 2017, 7, 7902–7907; (e) Romano, C.; Fiorito, D.; Mazet, C. Remote Functionalization of α,β-Unsaturated Carbonyls by Multimetallic Sequential Catalysis. J. Am. Chem. Soc. 2019, 141, 16983–16990.
- 15For selected examples on asymmetric bimetallic catalysis, see: (a) Sawamura, M.; Sudoh, M.; Ito, Y. An Enantioselective Two-Component Catalyst System: Rh-Pd-Catalyzed Allylic Alkylation of Activated Nitriles. J. Am. Chem. Soc. 1996, 118, 3309–3310; (b) Guan, X.-Y.; Yang, L.-P.; Hu, W. Cooperative Catalysis in Multicomponent Reactions: Highly Enantioselective Synthesis of γ-Hydroxyketones with a Quaternary Carbon Stereocenter. Angew. Chem. Int. Ed. 2010, 49, 2190–2192; (c) Ikeda, M.; Miyake, Y.; Nishibayashi, Y. Cooperative Catalytic Reactions Using Distinct Transition-Metal Catalysts: Ruthenium- and Copper-Catalyzed Enantioselective Propargylic Alkylation. Chem.-Eur. J. 2012, 18, 3321–3328; (d) Nahra, F.; Macé, Y.; Lambin, D.; Riant, O. Copper/Palladium-Catalyzed 1,4 Reduction and Asymmetric Allylic Alkylation of α,β-Unsaturated Ketones: Enantioselective Dual Catalysis. Angew. Chem. Int. Ed. 2013, 52, 3208–3212; (e) Smith, K. B.; Logan, K. M.; You, W.; Brown, M. K. Alkene Carboboration Enabled by Synergistic Catalysis. Chem.-Eur. J. 2014, 20, 12032–12036; (f) Jia, T.; Cao, P.; Wang, B.; Lou, Y.; Yin, X.; Wang, M.; Liao, J. A Cu/Pd Cooperative Catalysis for Enantioselective Allylboration of Alkenes. J. Am. Chem. Soc. 2015, 137, 13760–13763; (g) Li, J.; Lin, L.; Hu, B.; Lian, X.; Wang, G.; Liu, X.; Feng, X. Bimetallic Gold(I)/Chiral N,N’-Dioxide Nickel(II) Asymmetric Relay Catalysis: Chemo- and Enantioselective Synthesis of Spiroketals and Spiroanimals. Angew. Chem. Int. Ed. 2016, 55, 6075–6078; (h) Friis, S. D.; Pirnot, M. T.; Buchwald, S. L. Asymmetric Hydroarylation of Vinylarenes Using a Synergistic Combination of CuH and Pd Catalysis. J. Am. Chem. Soc. 2016, 138, 8372–8375; (i) Logan, K. M.; Brown, M. K. Catalytic Enantioselective Arylboration of Alkenylarenes. Angew. Chem. Int. Ed. 2017, 56, 851–855; (j) Saito, A.; Kumagai, N.; Shibasaki, M. Cu/Pd Synergistic Dual Catalysis: Asymmetric α-Allylation of an α-CF3 Amide. Angew. Chem. Int. Ed. 2017, 56, 5551–5555.
- 16(a) Sato, I.; Matsueda, Y.; Kadowaki, K.; Yonekubo, S.; Shibata, T.; Soai, K. Highly Enantioselective Asymmetric Autocatalysis of Pyrimidin-5-yl Alkanol Induced by Chiral 1,3-Disubstituted Hydrocarbon Allenes. Helv. Chim. Acta 2002, 85, 3383–3387; (b) Löhr, S.; Averbeck, J.; Schürmann, M.; Krause, N. Synthesis and Complexation Properties of Allenic Bipyridines, a New Class of Axially Chiral Ligands for Transition Metal Catalysis. Eur. J. Inorg. Chem. 2008, 552–556; (c) Pu, X.; Qi, X.; Ready, J. M. Allenes in Asymmetric Catalysis: Asymmetric Ring Opening of meso-Epoxides Catalyzed by Allene-Containing Phosphine Oxides. J. Am. Chem. Soc. 2009, 131, 10364–10365; (d) Cai, F.; Pu, X.; Qi, X.; Lynch, V.; Radha, A.; Ready, J. M. Chiral Allene- Containing Phosphines in Asymmetric Catalysis. J. Am. Chem. Soc. 2011, 133, 18066–18069.
- 17For review of bioactive cyclopentenones, see: (a) Simeonov, S. P.; Nunes, J. P. M.; Guerra, K.; Kurteva, V. B.; Afonso, C. A. M. Synthesis of Chiral Cyclopentenones. Chem. Rev. 2016, 116, 5744–5893; For selected examples of bioactive cyclopentenones, see: (b) Ockey, D. A.; Lewis, M. A.; Schore, N. E. A Short Synthesis of (±)-Tecomanine via a Pauson-Khand-Based Route. Tetrahedron 2003, 59, 5377–5381; (c) Berges, C.; Fuchs, D.; Opelz, G.; Daniel, V.; Naujokat, C. Helenalin Suppresses Essential Immune Functions of Activated CD4+ T Cells by Multiple Mechanisms. Mol. Immunol. 2009, 46, 2892–2901.
- 18(a) Lee, M.; Lee, Y.-J.; Park, E.; Park, Y.; Ha, M. W.; Hong, S.; Lee, Y.-J.; Kim, T.-S.; Kim, M.-H.; Park, H.-G. Highly Enantioselective Synthesis of 5-Phenyl-2-alkylprolines Using Phase-Transfer Catalytic Alkylation. Org. Biomol. Chem. 2013, 11, 2039–2046; (b) Xue, Z.-Y.; Xiong, Y.; Wang, C.-J. Catalytic Asymmetric Construction of Azabicyclo[2.2.1]heptanes Bearing Two Quaternary Stereogenic Centers via Silver(I)- Catalyzed 1,3-Dipolar Cycloaddition of Cyclic Azomethine Ylides. Synlett 2014, 25, 2733–2737; (c) Koizumi, A.; Kimura, M.; Arai, Y.; Tokoro, Y.; Fukuzawa, S.-I. Copper- and Silver-Catalyzed Diastereo- and Enantioselective Conjugate Addition Reaction of 1-Pyrroline Esters to Nitroalkenes: Diastereoselectivity Switch by Chiral Metal Complexes. J. Org. Chem. 2015, 80, 10883–10891; (d) Zheng, X.; Yang, W.-L.; Liu, Y.-Z.; Wu, S.-X.; Deng, W.-P. Enantioselective Synthesis of Tropanes via [3+3] Annulation of Cyclic Azomethine Ylides with Substituted 2-Vinylindoles and 2-Vinylpyrroles. Adv. Synth. Catal. 2018, 360, 2843–2853.
- 19(a) Xue, Z.-Y.; Song, Z.-M.; Wang, C.-J. Cu(I)/TF-BiphamPhos-Catalyzed Asymmetric Michael Addition of Cyclic Ketimino Esters to Alkylidene Malonates. Org. Biomol. Chem. 2015, 13, 5460–5466; (b) Li, C.-Y.; Yang, W.-L.; Luo, X.; Deng, W.-P. Diastereodivergent Asymmetric Michael Addition of Cyclic Azomethine Ylides to Nitroalkenes: Direct Approach for the Synthesis of 1,7-Diazaspiro[4.4]nonane Diastereoisomers. Chem.-Eur. J. 2015, 21, 19048–19057; (c) Koizumi, A.; Harada, M.; Haraguchi, R.; Fukuzawa, S.-I. Chiral Silver Complex- Catalyzed Diastereoselective and Enantioselective Michael Addition of 1-Pyrroline-5-carboxylates to α-Enones. J. Org. Chem. 2017, 82, 8927–8932.




