Chiral stationary phases and applications in gas chromatography
Dedicated to the pioneers of enantioselective chromatography on the occasion of Prof. Dr. Yoshio Okamoto's 80th birthday.
Abstract
Chiral compounds are ubiquitous in nature and play a pivotal role in biochemical processes, in chiroptical materials and applications, and as chiral drugs. The analysis and determination of the enantiomeric ratio (er) of chiral compounds is of enormous scientific, industrial, and economic importance. Chiral separation techniques and methods have become indispensable tools to separate chiral compounds into their enantiomers on an analytical as well on a preparative level to obtain enantiopure compounds. Chiral gas chromatography and high-performance liquid chromatography have paved the way and fostered several research areas, that is, asymmetric synthesis and catalysis in organic, medicinal, pharmaceutical, and supramolecular chemistry. The development of highly enantioselective chiral stationary phases was essential. In particular, the elucidation and understanding of the underlying enantioselective supramolecular separation mechanisms led to the design of new chiral stationary phases. This review article focuses on the development of chiral stationary phases for gas chromatography. The fundamental mechanisms of the recognition and separation of enantiomers and the selectors and chiral stationary phases used in chiral gas chromatography are presented. An overview over syntheses and applications of these chiral stationary phases is presented as a practical guidance for enantioselective separation of chiral compound classes and substances by gas chromatography.
1 INTRODUCTION
The chromatographic separation of stereoisomers and of enantiomers in particular is of extraordinary importance.1 The development of high performance chiral stationary phases (CSP) for the separation of enantiomers by gas chromatography (GC),2-7 high performance liquid chromatography (HPLC),8-26 supercritical fluid chromatography (SFC),27-30 and capillary electrochromatography (CEC)31-38 has completely replaced indirect separation methods, for example, the laborious derivatization of chiral compounds with enantiomerically pure chiral reagents to form diastereomeric compounds, due to their enormous and universal applications.39-41 Modern chiral stationary phases are often applicable to a broad spectrum of different chiral compound classes and allow rapid analytical determination of enantiomeric ratios (er). Furthermore, the assignment of absolute configurations in the case of known compounds and, especially, in the case of reaction upscale, also the preparative separation of enantiomers,42, 43 which may save time and resources in the development of drug leads compared to asymmetric synthesis, is feasible.
Chiral stationary phases are among the enabling technologies, as they have paved the road for scientific progress in many fields. These include asymmetric synthesis44 and catalysis45-49 asymmetric organocatalysis50-58; assignment of relative and absolute configurations59-61; high-throughput screenin62-64 of chiral compounds, medicinal chemistry, drug discovery, and challenging scientific questions about asymmetric autocatalysis65, 66; self-amplification of chirality67-71; or the homochirality in the context of the origins of life72-74or the enrichment of chiral compounds in extraterrestrial samples.75-79 Furthermore, chiral stationary phases also allow the investigation of physical organic properties,80, 81 such as supramolecular interactions82-85 or the dynamic behavior of interconverting enantiomers.86-97
The present review focuses on the design,92-102 development, and application of the most important chiral stationary phases for chiral GC. It has to be noted that the terms chiral GC and enantioselective GC are used interchangeably in the literature, despite that enantioselective GC is scientifically more appropriate, because a chiral stationary phase is not necessarily enantioselective. Because of the broader acceptance of chiral GC in literature, this term is used throughout this review.
Gil-Av et al.103 accomplished the first separation of enantiomers by GC in 1966. They utilized the amino acid derivative N-trifluoroacetyl-L-isoleucine lauryl ester 1 (Figure 1) as CSP and were able to separate enantiomers of the proteinogenic α-amino acids, derivatized as the N-trifluoroacetyl alkyl esters of alanine, valine and leucine on a glass capillary column (100 m × 0.25 mm inner diameter [i.d.]).
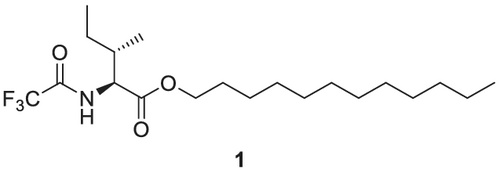
The recognition principle is based on complementary hydrogen bonding, which is also known from selective peptide-peptide (β-sheet in the secondary peptide structure) interactions. Furthermore, the principle of a 3-point interaction104 necessary for a successful enantioselective recognition was realized, which was pointing the way to more efficient CSPs.
The CSP N-trifluoroacetyl-L-isoleucine lauryl ester was of central importance in the investigation of the enantiomer composition of extraterrestrial material obtained by the Apollo program.105 Surprisingly, no amino acids were found in lunar samples from the Sea of Tranquility.
In 1977, Frank et al.106-108 permanently bound the L-valine diamide selector to polydimethylsiloxane (Chirasil-Val) and thus developed a highly enantioselective CSP, especially for amino acid analysis. Schurig and Gil-Av,109 Schurig,110 and Schurig and Bürkle111 extended the spectrum of CSPs to metal complexes, which is based on the observation that chiral β-diketonate complexes are excellent chiral shift reagents in NMR spectroscopy.112-114
With the development of CSPs based on cyclodextrin (CD) derivatives, this field has evolved enormously fast, as supramolecular host-guest complexes eliminated the need for directional transient bonding. This opened the entire spectrum of separation of non-functionalized molecules.
2 SELECTOR CLASSES FOR CHIRAL RECOGNITION IN GAS CHROMATOGRAPHY
In general, enantiomeric separation is based on the principle of “chiral recognition,” in which adducts of the enantiomers and the chiral selectors of the stationary phase are formed. This results in the formation of transient diastereomeric complexes. The advantage of this technique is that these equilibria are adjusted in each theoretical separation plate of a separation column, because high separation efficiencies with 1,000 to 5,000 theoretical plates per meter GC capillary column can be achieved and thus excellent separations can be observed even with small energetic differences between the formed transient diastereomeric complexes between the enantiomers and the selector.
As already mentioned, chiral recognition can be achieved by 3-point interaction between selector and selectand (cf. Figure 2). This can be realized by binding interactions (hydrogen bonds, charge-transfer interactions, and coordination) but also by non-binding interactions, such as steric hindrance.
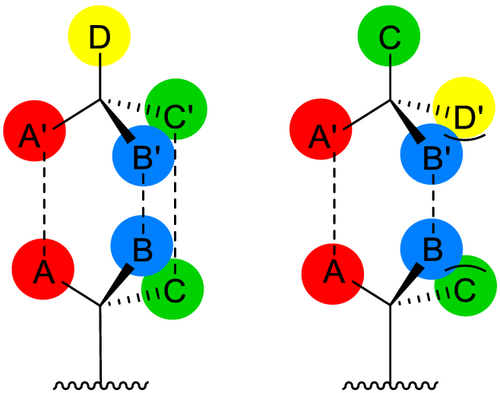
The two transient diastereomeric adducts that form differ in their thermodynamic properties, resulting in different retention times in chromatographic separation.
Important advantages of the separation of enantiomers by gas chromatography are high efficiency, sensitivity, and rapid separation. Furthermore, coupling techniques, such as GC-MS, are accessible and allow unambiguous assignment of analytes. Using selected ion monitoring (SIM) mode in GC-MS, even traces of enantiomers can be easily detected and identified.
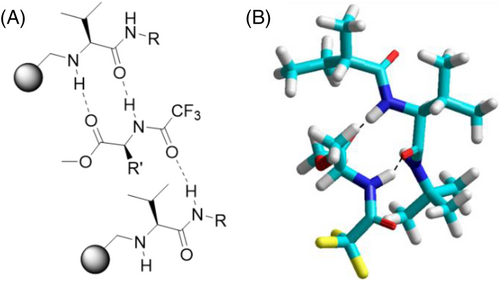
2.1 Chiral recognition by hydrogen bonding
Hydrogen bonding is highly efficient for interaction between the selector and the selectand/analyte molecule (Figure 3). This recognition principle is used in highly versatile diamide CSPs like the N-trifluoroacetyl-L-isoleucine lauryl ester developed by Gil-Av et al.103 or Chirasil-Val.106-108
2.2 Chiral recognition by host-guest interactions
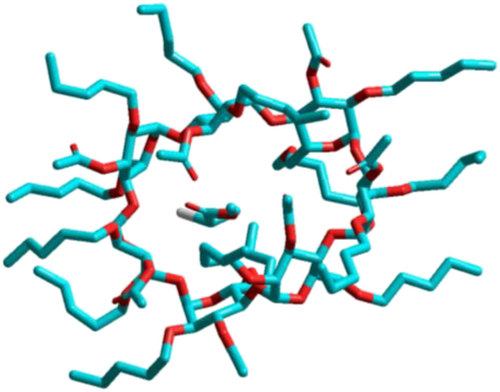
Host-guest interactions (Figure 4) are highly versatile in chiral recognition because a supramolecular chiral host for example CDs115 is able to interact through multiple non-covalent interactions. Experimental investigations of interactions between enantiomers and chiral hosts such as (derivatized) CDs have been performed by spectroscopic techniques and supported by molecular dynamics (MD) simulations and molecular modeling.116-118 The advantage is that chiral recognition is possible also for non-functionalized chiral molecules, which even makes the separation of chiral alkanes feasible. These types of host-guest interactions can be combined with hydrogen bonding by introducing hydrogen-bond-acceptor or hydrogen-bond-donor groups, respectively. This has been demonstrated for the decoration of resourcinarenes with N-acetyl-L-valine-tert-butylamide moieties.119 Resourcinarenes consist of cyclic methylene bridged resorcin tetramers, which are similar to the structure of calixarenes. Recent developments include metal organic frameworks (MOF) with chiral recognition sites.120
2.3 Chiral recognition by analyte complexation to chiral metal complexes
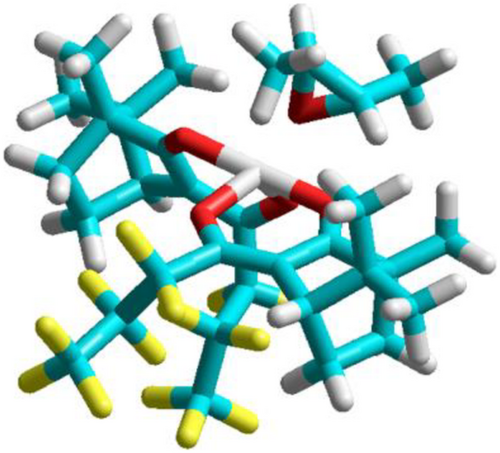
The Zeise-salt121 where the strong π-acid ethylene is coordinated to platinum represents an example for organic compounds where ligands with free electron pairs or π-bonds are coordinated to the metal center. The trick is to find chiral ligands which coordinate very well to the metal and are not substituted by the coordinating analyte molecule. β-diketonate complexes are well suited and can be prepared from Nature's chiral pool of terpenes.122, 123 Various metal ions can be coordinated. The most versatile phases are based on nickel (II) and europium (III) complexes. In Figure 5, an interaction of (S,S)-2,3-dimethyloxirane124 with the selector nickel (II)-bis [(1R)-3-(heptafluorobutyryl)-camphorate] is shown.125
3 PRACTICAL ASPECTS FOR CHIRAL GAS CHROMATOGRAPHY
3.1 Coating of fused-silica capillaries with chiral stationary phases
Most GC-separation capillaries coated with chiral stationary phases are commercially available; however, there are several non-commercially available CSPs, which can be coated onto bare fused silica capillaries. This practical guide is also intended for proper treatment of capillaries and to keep high separation efficiencies over their lifetime.
Fused silica capillary columns, 12.5 to 50 m, typically with an inner diameter of 0.25 mm, must first be prepared for coating with (chiral) stationary phases. For this purpose, the capillaries prepared on capillary cages are first dehydrated at 260°C in a weak carrier gas stream for 48 h and used directly for coating with the corresponding chiral stationary phase by the static method126 at room temperature. It must be emphasized here that some very nonpolar stationary phases, for example, the Lipodex phases, which were originally developed for pyrex glass capillaries, require silanization of the capillary surface. This can be done either via silanization reagents or by coating with a hydridomethyldimethylpolysiloxane with up to 10% hydrido groups and a layer thickness of 5–10 nm.
The capillary is first filled with this solution using a coating apparatus, and the end of the capillary is sealed. The use of silicone, which is filled into a pipette in a soft form and in which the capillary is inserted, has proven to be favorable. Here, it is important that any bubbles are removed by further rinsing with coating solution and pushing the capillary further into the silicone. The solvent is now removed by applying a vacuum to the filling side, and the progress of the coating can be followed visually. Typically, this evaporation is facilitated with a water bath, because the fused-silica capillaries have excellent heat transfer properties. If the solvent front is disrupted during solvent removal, the capillary must be filled again.
Conditioning of the coated capillaries is performed using a temperature program (50°C to 180°C, 1°C/min), and the temperature is then maintained for 24 h.
For thermal immobilization of Chirasil phases, the conditioned capillaries are left at 220°C for 48 h in a very weak carrier gas stream (about 50–80 bubbles/min in ether). Immobilized phases, unlike dissolved phases, can be purged. This is also quite useful if it is desired to regenerate immobilized phases from time to time. To do this, the capillary columns are rinsed with about 50 ml of anhydrous diethyl ether and conditioned again.
The coating of the capillaries can be validated by acquiring test chromatograms with test mixtures. For quality purposes, test mixtures, for example, the Schurig test128 (α-pinene, trans- and cis-pinane, rac- and meso-2,3-butanediol, γ-valerolactone, 1-phenylethylamine, 1-phenylethanol, and 2-ethylhexanoic acid), should be used periodically to document changes in retention or selectivity.
4 CHIRAL STATIONARY PHASES: SYNTHESIS AND APPLICATION IN GAS CHROMATOGRAPHY
4.1 Chiral stationary phases with diamide-selectors
4.1.1 Chirasil-Val
Chirasil-Val is the most prominent diamide selector with superior chiral recognition properties of all canonical amino acids, derivatized as N(O,S)-pentafluoropropionyl/isopropyl esters. In general, the problem of diamides is that these compounds tend to form insoluble solids because of the favored formation of self-association complexes and typically high polarity of the two diamide bonds. To use a valine-diamide selector as a CSP in GC, it is mandatory that the diamide selector is in a liquid, supercooled state. This is difficult to achieve, and therefore, Gil-Av et al.103 prepared the lauryl ester, which prevents the agglomeration and precipitation. Another strategy was developed by Frank et al.106-108 They utilized polysiloxanes as inert polymer backbone and attached the selector to a functionalized copolymer, which circumvents formation of solid agglomerates. Another advantage of the immobilization strategy is that these immobilized phases are typically more stable and give better results in GC-MS coupling, because of reduced column bleeding of impurities or fragments formed during synthesis. This CSP is synthesized from L-valine 2 by N-Boc protection using di-tert-butyl dicarbonate, followed by amide formation with tert-butylamine. After deprotection of the N-Boc-L-valine-tert-butylamide 4 with TFA, the resulting -L-valine-tert-butylamide is coupled to the polymeric backbone copolymers of poly[(2-carboxylpropyl)methylsiloxane]/polydimethylsiloxane 6 using N,N-dicyclohexylcarbodiimide (DCC) (Scheme 1).
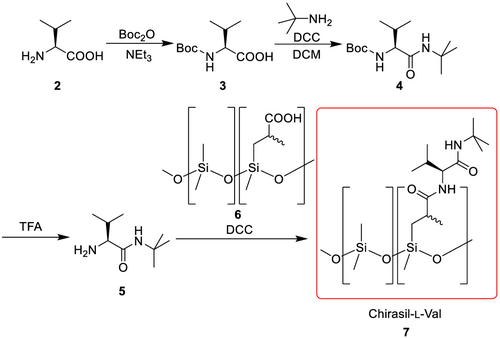
Chirasil-Val 7 can be used between 70°C and 240°C. A broad range of chiral compounds can be separated such as amino acids (Table 1), hydroxy acids, alcohols, amines, and biphenyl derivatives. As expected, L-amino acids elute after the D-isomer, because of the matching L-amino acid/L-amino acid interactions.
| Compound | Separation temperature T (°C) | Separation factor α | First eluted |
|---|---|---|---|
| Alanine A | 100 | 1.129 | D |
| Valine V | 100 | 1.117 | D |
| Isoleucine I | 100 | 1.158 | D |
| Leucine L | 100 | 1.222 | D |
| Threonine T | 100 | 1.069 | D |
| Serine S | 120 | 1.052 | D |
| Proline P | 100 | 1.005 | D |
| Cysteine | 120 | 1.060 | D |
| Methionine M | 140 | 1.079 | D |
| Phenylalanine F | 140 | 1.086 | D |
| Ornithine Orn | 170 | 1.081 | D |
| Lysine K | 170 | 1.065 | D |
| Aspartic acid D | 120 | 1.031 | D |
| Glutamic acid E | 140 | 1.108 | D |
| Tryptophane W | 170 | 1.078 | D |
| Tyrosine Y | 170 | 1.024 | D |
- Note: Twenty meter Schott glass capillary column, 0.30 mm i.d., coated with L-Chirasil-Val.106
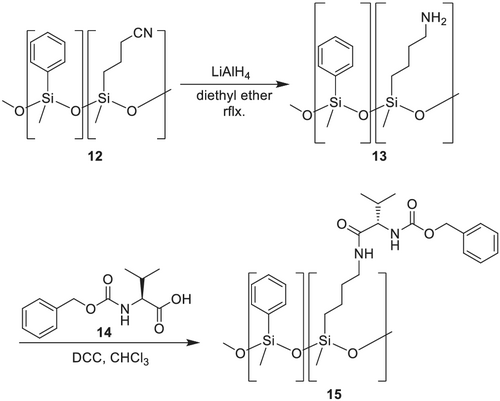
König et al.129 developed similar CSPs for the separation of amines, amino acids, carbohydrates, and hydroxy carboxy acids. They used cyanoethyl polysiloxane (XE-60 8),129, 130 which was hydrolyzed to the corresponding carboxy acid and cyanopropyl polysiloxane (OV-225 12), which was converted into the corresponding amine by reduction with LiAlH4. This reduction of OV-225 is a limiting step because it can result in an insoluble gum, due to partial hydrolysis and resulting cross-linking by reductive amination. They replaced the tert-butylamide by (S)- or (R)-1-phenylethylamide in the XE-60-Val phase (Scheme 2).
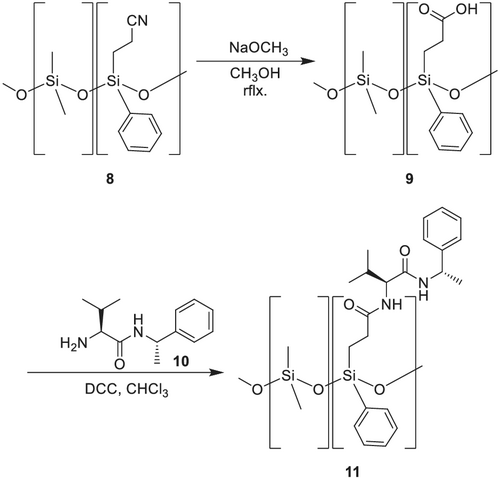
The remarkable property of the CSP XE-60-L-valine-(S)-1-phenylethylamide 11 is that carbohydrate enantiomers as trifluoroacetates or trifluoroacetate/methylglycosides can be separated.131
With the reduced OV-225 12, inverse CSPs were prepared using benzyloxycarbonyl-L-valine 14 and -L-leucine (Scheme 3).131
4.2 Chiral stationary phases with cyclodextrin-selectors
Due to their ability to form selective inclusion complexes, CDs were considered as a stationary phase in GC early on.132, 133 Kościelski et al.134, 135 and Smolková et al.136, 137 and Mráz et al.138 made pioneering contributions to this field, investigating the interactions of packed CD columns with chiral analytes such as α- and β-pinene134, 135 and various nonchiral analytes.136-138 High separation efficiencies were finally achieved by coating capillary columns with liquid derivatized CDs and CDs dissolved in polysiloxane polymer.
These selectively alkylated/acylated CDs exhibit high enantioselectivity and are versatile selectors broadly used for the separation of enantiomers by gas chromatography (GC).139-142
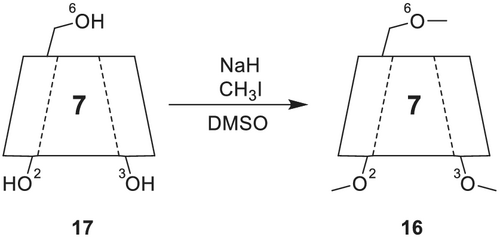
4.2.1 Heptakis(2,3,6-tri-O-methyl)-β-cyclodextrin (Permethyl-β-cyclodextrin)
Permethylated β-CD 16 (commercially available as, e.g., Astec CHIRALDEX B-PM; Supelco β-DEX 110; Supelco β-DEX 120) (Figure 6) is a widely used selector, because of the straightforward preparation (Scheme 4) and the excellent enantioselectivity for a broad range of analytes. Initially, undiluted permethylated β-CD was directly coated on glass capillary column and used in the “supercooled liquid state” as a CSP.143
However, such supercooled liquid state columns are difficult to handle, and spontaneous crystallization accompanied with a loss of enantioselectivity can occur. To overcome this problem of melting points and phase transitions, Schurig and Nowotny144 dissolved permethylated β-CD 16 in semipolar polysiloxanes such as OV-1701. This combination results in a reproducible chemical selectivity and chromatographic efficiency.144 The approach of diluting selectors is broadly used, and chiral GC columns are commercially available from major chromatographic suppliers.145
4.2.2 Heptakis(2,3,6-tri-O-methyl)-β-cyclodextrin immobilized to hydrido dimethyl polysiloxane (Chirasil-β-Dex)
Similar to Chirasil-Val 7, where the immobilization prevents self-association and precipitation of the chiral selector, immobilization of permethylated β-CD 16 via a polymethylene linker to polydimethylsiloxane to yield a chiral polysiloxane-containing CD (Chirasil-β-Dex) 18 was a logical design step.146-148 Furthermore, Chirasil-β-Dex can be thermally immobilized on the inner capillary wall, which makes purging with solvents and use in liquid separations feasible. The first synthesis of Chirasil-Dex utilized trimethylene,147 pentamethylene,148 and octamethylene148, 149 spacers juxtaposed between the permethylated CD and the polysiloxane backbone (Scheme 5). It was assumed that the trimethylene spacer was attached at O-6 position of the CD, because using sodium hydroxide in dimethylsulfoxide should functionalize the more acidic primary hydroxy groups of β-CD at room temperature yielding the O-6 derived ethers. During subsequent modification of the reaction protocols as well as purification steps towards the access to the elongated mono-oct-7-enyl ether of β-CD 19,148 a competitive O-2-alkenylation can be envisioned for Chirasil-Dex.148 A detailed GC-MS analysis of degradation products of permethylated mono-O-pent-1-enyl-β-CD obtained in dimethyl sulfoxide (DMSO) in the presence of sodium hydroxide indicated that the ether was predominantly (96%) formed at the O-2 position.149
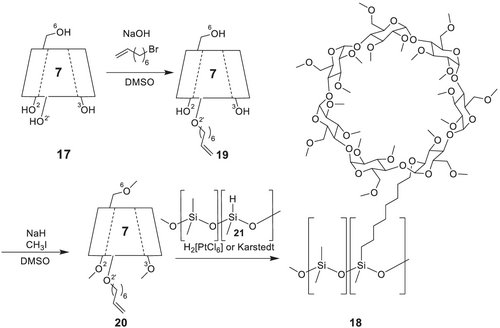
However, the unambiguous interpretation of NMR spectra especially for mono-substituted CD derivatives is extremely complicated due to the loss of Cn-symmetry, which results in signal overlapping and allows only estimations about purity and substitution pattern. In an extensive preparative work, all three regioisomers O-2, O-3 and O-6-Chirasil-Dex were synthesized and characterized.150 Because the regioselective O-2-Chirasil-Dex columns showed the highest separation factors α for almost all tested enantiomers, the non-regioselective Chirasil-Dex columns, originally formulated as O-6, but then revised as a mixture of O-2- and O-6-Chirasil-Dex with a dominance of O-2-Chirasil-Dex are most likely operating at optimum enantioselectivities in various applications of different chromatographic techniques.148 The differences between O-2, O-3, and O-6-Chirasil-Dex regioisomers appear not to be pronounced enough to warrant the rather tedious synthetic pathway to selected regioisomers.
Chirasil-β-Dex is one of the most versatile CSPs and is recommended as a starting point for developing and optimizing chiral separations, because a broad range of compounds, including compounds with stereogenic nitrogen (Tröger's base,151 aziridines, diaziridines, etc.),152-155 can be separated with excellent separation factors α and resolution R (Table 2).
| Compound | Separation temperature T (°C) | Separation factor α |
|---|---|---|
| N-TFA-Ala-iso-propyl ester | 80 | 1.184 |
| N-TFA-Ser-iso-propyl ester | 90 | 1.184 |
| N-TFA-Cys-iso-propyl ester | 100 | 1.105 |
| N-TFA-Phe-iso-propyl ester | 120 | 1.022 |
| 1-(2-methylphenyl) ethanol | 110 | 1.343 |
| 1-(2-bromophenyl) ethanol | 130 | 1.440 |
| 1-(3-methylphenyl) ethanol | 100 | 1.124 |
| 1-phenylethanol | 100 | 1.140 |
| mandelic acid propyl ester | 130 | 1.100 |
| 3,5-dimethyl-2-cyclohexen-1-on | 90 | 1.110 |
| 2,4-dimethylheptene | 40 | 1.034 |
| 3-methylheptane | 40 | 1.034 |
| 1,2-di-tert-butyldiaziridine | 80 | 1.440 |
| 1-isopropyl-2-n-propyldiaziridine | 100 | 1.180 |
| 1,2-diisopropyldiaziridine | 100 | 1.240 |
| 3,4-di-tert-butyl-1,3,4-oxadiazolidine | 85 | 1.170 |
4.2.3 Heptakis(2,6-di-O-methyl-3-O-pentyl)-β-cyclodextrin
The synthesis and application of the chiral selector heptakis(2,6-di-O-methyl-3-O-pentyl)-β-CD 21 (commercially available as, e.g., MEGA-DEX DMP-Beta) (Figure 7) were almost simultaneously reported by König et al.,156 who investigated its potential for the separation of chiral compounds in essential oils, and Bicchi et al.,157 who focused on investigating column performance parameters such as reproducibility.
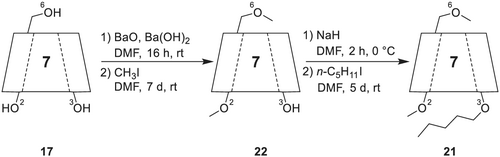
The selector is prepared by deprotonating lyophilized β-CD with a barium oxide (BaO)/barium hydroxide (Ba[OH]2) mixture, subsequent addition of methyl iodide and stirring of the reaction mixture for 1 week (Scheme 6).158 Afterwards, the doubly methylated β-CD is subjected to deprotonation with sodium hydride (NaH), addition of pentyl iodide, and is subsequently stirred for 5 days. Bicchi et al.157 report a slightly altered protocol, employing different reaction times and a pentyl bromide/pentyl iodide mixture for the pentylation step.
In the investigation of a test mixture Bicchi et al.157 noted the better reproducibility and high performance consistency of CSPs based on heptakis(2,6-di-O-methyl-3-O-pentyl)-β-CD 21 dissolved in OV-1701 polysiloxane compared to their permethylated β-CD analogs. Selected results of an extensive analyte screening on heptakis(2,6-di-O-methyl-3-O-pentyl)-β-CD phases by König158 are shown in Table 3.
| Compound | Separation temperature T (°C) | Separation factor α |
|---|---|---|
| Valine V | 80 | 1.037 |
| Proline P | 100 | 1.075 |
| Methionine M | 100 | 1.023 |
| Phenylalanine F | 110 | 1.031 |
| Aspartic acid D | 105 | 1.041 |
| Glutamic acid E | 110 | 1.050 |
| 5-Phenylhydantoina | 175 | 1.287 |
| α-Pineneb | 70 | 1.057 |
| Limonene | 70 | 1.070 |
| Campheneb | 70 | 1.087 |
| 1-tert-Butyl-1,2 cyclooctadiene | 80 | 1.411 |
| 2-Hydroxyoctanoic acid (OMe)c | 80 | 1.602 |
| 2-Hydroxydodecanoic acid (OMe)c | 125 | 1.153 |
| 1-Phenyl-3-butanolc | 95 | 1.104 |
| 1-Phenylethanol (TFA) | 90 | 1.111 |
| 1-Octen-3-ol | 98 | 1.049 |
| 2-Octanol | 90 | 1.016 |
| Ibuprofen (OMe) | 135 | 1.042 |
| Malic acid (OMe) | 100 | 1.149 |
| Mandelic acid (OMe)c | 105 | 1.120 |
| Myrtenold | 105 | 1.078 |
| Menthold | 100 | 1.092 |
| Linalool | 95 | 1.082 |
| Isoborneol | 95 | 1.030 |
| Phenyloxirane | 105 | 1.127 |
| (E)-1,2-Diphenyloxiranec | 135 | 1.108 |
| Citronellald | 75 | 1.027 |
| Menthoned | 90 | 1.098 |
| Carvoned | 100 | 1.068 |
- Note: Amino acids were derivatized as N-trifluoroacetyl amino acid methyl esters. Trifluoroacetylated alcohols are marked by TFA in brackets and methylated carboxylic acids are marked by OMe in brackets. Twenty five meter fused silica capillary column, 0.25 mm i.d., coated with heptakis(2,6-di-O-methyl-3-O-pentyl)-β-CD 21.159
- a Column length: 4.5 m.
- b Column length: 50 m.
- c Column length: 8 m.
- d Column length: 30 m.
The results show that CSPs based on the heptakis(2,6-di-O-methyl-3-O-pentyl)-β-CD 21 selector exhibit high enantioselectivity towards carboxylic acids, hydroxy carboxylic acids, epoxides, terpenes, and terpene alcohols while exhibiting more moderate separation factors for compound classes such as amino acids.
4.2.4 Hexakis-(2,3,6-tri-O-pentyl)-α-cyclodextrin
Hexakis-(2,3,6-tri-O-pentyl)-α-CD 23 (commercially available as, e.g., Lipodex A)159, 160 (cf. Figure 8) is a CD-based CSP for gas chromatography used for the separation of a broad range of chiral compounds such as carbohydrates, polyols, diols, hydroxy acid esters, (epoxy-) alcohols, glycerol derivatives, spiroketals, ketones, and alkylhalogenides. Most carbohydrates, derivatized as trifluouroacetylated carbohydrates, show excellent separation factors α.
The use of CDs as a stationary phase allows a high number of chiral centers to be introduced, leading to increased enantioselectivity. CDs exhibit high thermal stability operating at temperatures from 40°C up to 220°C.
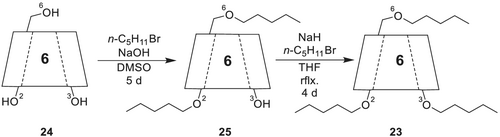
Hexakis-(2,3,6-tri-O-pentyl)-α-CD 23 is synthesized (Scheme 7) from α-CD 24 by pentylation of the O-2 and O-6 positions using n-pentyl bromide and powdered sodium hydroxide (NaOH) in anhydrous DMSO. To increase the overall yield, the base and n-pentyl bromide are repeatedly added every day. The hexakis-(2,3,6-tri-O-pentyl)-α-CD 23 is obtained after stirring for 5 days at room temperature and flash column chromatography. In a consecutive step, the pentylation of the O-3 positions is achieved with sodium hydride and n-pentyl bromide under refluxing conditions in tetrahydrofuran (THF) in 4 days.
The previously mentioned excellent separation factors α for carbohydrates are shown in Table 4. The concept of a close structural relationship between chiral selector and chiral substrate proves advantageous here. The polarity of hexakis(2,3,6-tri-O-pentyl)-α-CD 23 is comparable with the polarity of the methyl-phenyl polysiloxane OV-17 phase.
| Compound | Separation temperature T (°C) | Separation factor α | First eluted |
|---|---|---|---|
| α-Glucose | 100 | 1.119 | L |
| β-Glucose | 100 | 1.140 | L |
| α-Galactose | 100 | 1.070 | L |
| β-Galactose | 130 | 1.080 | L |
| α-Allose | 120 | 1.171 | D |
| β-Allose | 120 | 1.064 | D |
| α-Mannose | 100 | 1.000 | - |
| β-Mannose | 100 | 1.110 | L |
| α-Gulose | 120 | 1.000 | - |
| β-Gulose | 120 | 1.043 | D |
| α-Talose | 110 | 1.099 | D |
| β-Fucose (Furanoside) | 100 | 1.039 | L |
| α-Methylgalactoside | 100 | 1.091 | D |
| α-Methylglucoside | 100 | 1.035 | L |
| α-Methylmannoside | 100 | 1.051 | L |
| α-Methylidoside | 90 | 1.040 | D |
| α-Methylriboside | 110 | 1.075 | D |
| Sorbitol | 100 | 1.042 | D |
| Mannitol | 90 | 1.019 | D |
| Arabinitol | 100 | 1.175 | D |
| 1,5-Anhydrofucitol | 80 | 1.035 | D |
| 1,5-Anhydrolyxitol | 80 | 1.064 | D |
| 1,5-Anhydroarabinitol | 80 | 1.074 | D |
In addition, hexakis-(2,3,6-tri-O-pentyl)-α-CD 23 was used to separate enantiomers of a number of amino acids, which are listed in Table 5. D-amino acids are eluted prior to the L-amino acids.
| Compound | Separation temperature T (°C) | Separation factor α | First eluted |
|---|---|---|---|
| Threonine | 95 | 1.074 | D |
| Alanine | 80 | 1.036 | D |
| Phenylalanine | 130 | 1.028 | D |
4.2.5 Hexakis-(3-O-acetyl-2,6-di-O-pentyl)-α-cyclodextrin
Hexakis-(3-O-acetyl2,6-di-O-pentyl)-α-CD 26 (commercially available as, e.g., Lipodex B)159 (cf. Figure 9) is another example of a substituted CD-based CSP. In its application range between room temperature and 200°C, it is used for the separation of lactones, diols, cyclic carbonates, amino alcohols, aldols (O-TFA), and glycerol derivatives.
For the synthesis of hexakis-(3-O-acetyl2,6-di-O-pentyl)-α-CD 26163, 164 (Scheme 8), α-CD is dissolved in anhydrous DMSO and is stirred with sodium hydride and n-pentyl bromide for 5 days. The resulting hexakis-(2,6-di-O-pentyl)-α-CD is purified by column chromatography and then acetylated in a subsequent step with acetic anhydride, triethylamine, and 4-N-dimethylamino pyridine (DMAP) under refluxing THF for 72 h yielding hexakis-(3-O-acetyl-2,6-di-O-pentyl)-α-CD 26.
The separation of lactones, without previous derivatization, is summarized in Table 6 and shows good separation factors α for hexakis-(3-O-acetyl-2,6-di-O-pentyl)-α-CD 26.
| Compound | Separation temperature T (°C) | Separation factor α |
|---|---|---|
| 2-Methylbutyrolactone | 120 | 1.106 |
| 2-Ethylbutyrolactone | 120 | 1.063 |
| 2-n-Propylbutyrolactone | 120 | 1.031 |
| 2-n-Pentylbutyrolactone | 120 | 1.036 |
| 2-Methylvalerolactone | 120 | 1.026 |
| 3-Ethylvalerolactone | 140 | 1.035 |
| (E)-4-n-Butyl-3-methylbutyrolactone | 140 | 1.060 |
| (Z)-4-n-Butyl-3-methylbutyrolactone | 140 | 1.021 |
| (E)-3,4-DimethyIbutyrolactone | 140 | 1.121 |
| (Z)-3,4-Dimethylbutyrolactone | 140 | 1.094 |
| 4-Methylbutyrolactone | 150 | 1.108 |
| 4-Ethylbutyrolactone | 150 | 1.104 |
| 4-n-Propylbutyrolactone | 150 | 1.048 |
| 4-n-Butylbutyrolactone | 150 | 1.048 |
| 4-n-Pentylbutyrolactone | 150 | 1.066 |
| 4-n-Hexylbutyrolactone | 170 | 1.035 |
| 4-n-Octylbutyrolactone | 170 | 1.040 |
| 4-n-Decylbutyrolactone | 170 | 1.044 |
It is worth noting that the enantioselective properties of α- and β-CDs with the same substitution pattern (3-O-acetyl and 2,6-di-O-pentyl; hexakis-(3-O-acetyl-2,6-di-O-pentyl)-α-CD and heptakis(3-O-acetyl-2,6-di-O-pentyl)-β-CD, respectively) show remarkable differences.
4.2.6 Heptakis(2,3,6-tri-O-pentyl)-β-cyclodextrin
Heptakis(2,3,6-tri-O-pentyl)-β-CD (commercially available as, e.g., Lipodex C) 27163 (cf. Figure 10) as perpentylated β-CD phase shows a particularly remarkable enantioselectivity despite its similar structure compared to other selectors. Some previously inseparable alcohols can be separated on this phase, as well as cyanohydrins and carbohydrates. One example is the pheromone grandisol 28 (Figure 11), whose hydroxy group is bridged to the chiral center by a C2 unit. Also, many olefin enantiomers and alkyl halides can be separated with the perpentylated β-CD phase with thermal stability above 200°C.165
Heptakis(2,3,6-tri-O-pentyl)-β-CD 27 as perpentylated β-CD was obtained (Scheme 9) by a reaction of β-CD with n-pentyl bromide and powdered NaOH in anhydrous DMSO.
After enzymatic preparation in high enantiomeric ratio, particular attention should be paid to cyanohydrins 29 (Figure 11) as their enantiomeric purity is of great importance as precursors of amino and hydroxyl acids.166
Despite the range of possibilities to separate trifluoroacetylated carbohydrates chromatographically via CD phases, the best separation of 6-deoxy sugars is achieved on perpentylated β-CD 27.
Smaller homologs of α-hydroxy acid methyl esters can only be separated with alkylated CDs such as heptakis(2,3,6-tri-O-pentyl)-β-CD 27, whereas isopropyl urethanes of α-hydroxy acid methyl esters can be separated well on chiral polysiloxane phases. In this case, the hydroxy and methoxy group in 3- and 4-position of the aromatic ring of mandelic acid 30 (Figure 11) did not affect the successful separation of its enantiomers.
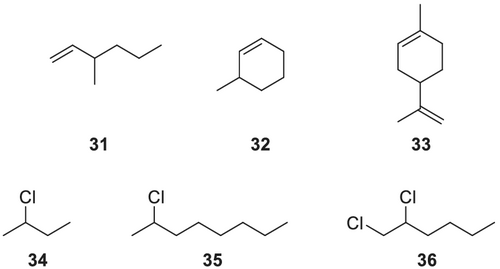
In addition to the above-mentioned, Kościelski et al.165 have already described the enantioselective interaction of unmodified α-CD with olefins and saturated hydrocarbons in β-pinene and its hydrogenation products. König et al.129 have found that by using perpentylated β-CDs 27 many other chiral olefins and dienes can also efficiently be separated. Nevertheless, structural changes are the limiting factor in the recognition of chiral olefins. 3-Methyl-1-hexene 31 (Figure 12) and 3-methyl-cyclohexene 32 (Figure 12) can be separated excellently, whereas no satisfying separation could be achieved for terpenes such as limonene 33 (Figure 12), for example.
In general, the size of the macrocycle in pentylated α- and β-CD affects the strength of the interaction with the substrate, and α- and β-CD therefore have different optima.
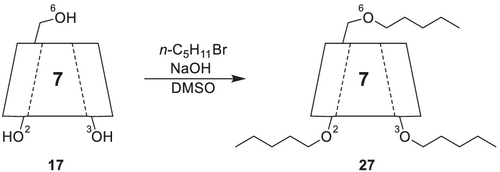
Finally, a very high enantioselectivity of heptakis(2,3,6-tri-O-pentyl)-β-CD 27 is observed towards chiral chloro- and bromoalkanes as well. The enantiomers of 2-chlorobutane 34 up to 2-chlorooctane 35 are fully separated with decreasing α-values (Figure 12). The more elongated the chain length is, the more decreased α-values are obtained. Furthermore, the disubstituted 1,2-dibromohexane 36 (Figure 12) could also be resolved, but the higher homologs 1,2-dibromoheptane and 1,2-dibromoctane were no longer separable.165
4.2.7 Heptakis(3-O-acetyl-2,6-di-O-pentyl)-β-cyclodextrin
Heptakis(3-O-acetyl-2,6-di-O-pentyl)-β-CD (commercially available as, e.g., Lipodex D) 37167 (cf. Figure 13) is a frequently used CSP in capillary gas chromatography equipped with broad applicability. As a key property, a high enantioselectivity towards trifluoro-acetylated α- and β-chiral amines, amino alcohols, α- and β-amino acid esters, and cyclic trans-diols is observed. Furthermore, heptakis(3-O-acetyl-2,6-di-O-pentyl)-β-CD 37 provides each user with a wide operating temperature range up to 200°C. One of the first examples for a heptakis(3-O-acetyl-2,6-di-O-pentyl)-β-CD 37 application was demonstrated by König et al.162, 168 They have shown that CDs display a high enantioselectivity after introduction of hydrophobic residues into the macrocyclic molecule.162, 164, 168
In this context, perpentylated α-CD has been used to separate the enantiomers of a large number of hydroxyl compounds such as carbohydrates, methyl glycosides, polyols, triols, diols, amino alcohols, and even alcohol after trifluoro acetylation at a relatively low column temperature. Hydrogen bonding interaction is supposed to be mainly responsible for enantiomeric resolution on chiral diamide stationary phases.169
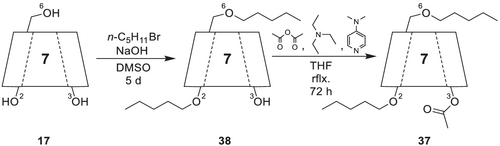
For the synthesis of heptakis(3-O-acetyl-2,6-di-O-pentyl)-β-CD 37, (Scheme 10) β-CD 17 is subjected to pentylation with sodium hydroxide and n-pentyl bromide in DMSO according to the method of Ciucanu and Kerek.164 The resulting heptakis(2,6-di-O-pentyl)-β-CD) 38 can be further acetylated with acetic anhydride, trimethylamine, and DMAP by boiling the solution for 72 h. After removing the solvent, the residue is extracted with tert-butyl methyl ether and purified by flash column chromatography on silica gel.
König's158 investigation has shown that many functional groups of the cyclodextrin molecules provide a range of possibilities for further modification. Characteristics such as good solubility in organic solvents, a low melting point, and high temperature stability have been obtained by the introduction of alkyl substituents. Since the hydroxyl groups show a difference in reactivity in positions 2, 3, and 6 of the anhydroglucose residues, it is generally possible to install different types of substituents to the molecule. As an example, upon pentylation of the hydroxyl groups in the positions 2 and 6, the remaining and less reactive hydroxy groups in position 3 can be acetylated. Hence, possibilities for weak hydrogen bonding or dipole-dipole interaction can be introduced without dramatic change of the hydrophobic character of the perpentylated CD. As a result of the above-mentioned approach, a strong increase in enantioselectivity towards substrates with the ability to form hydrogen bonds is observed in the case of the 3-acetyl-2,6-di-pentyl derivative of β-CD. This kind of modification causes extraordinarily large separation factors for amines and amino compounds with additional functional groups. In Figure 14, six representative examples for such an enantiomer separation are given.
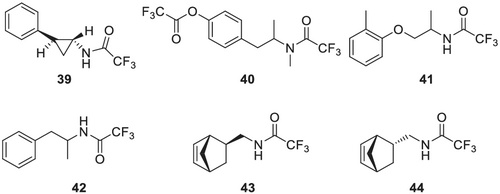
It is worth mentioning that some of the separations described are not feasible on other CSPs. For example, the separation of the amphetamine derivative 42, pholedrine 40 or the aminomethyl-norbornenes 43 and 44 with the center of chirality in β-position to the functional group.
In addition to amino compounds, also a high enantioselectivity is observed towards some diols such as propane-1,2-diol or butane-1,3-diol.
4.2.8 Octakis(3-O-butyryl-2,6-di-O-pentyl)-γ-cyclodextrin
Octakis(3-O-butyryl-2,6-di-O-pentyl)-γ-CD (commercially available as, e.g., Lipodex E and Astec CHIRALDEX G-BP)170 45 (cf. Figure 15) is one of the most versatile CSPs and separates a broad range of chiral compounds, that is, most of the canonical amino acids and some unusual amino acids, α- and β-hydroxy carboxylic acids, alcohols, diols, triols, ketones, bicyclic and tricyclic acetals, amines, alkyl halogen compounds, ethers, lactones, and chiral cyclopropanes. In particular, most amino acids, derivatized as N-trifluoroacetyl amino acid methyl esters, where in most cases the D-α-amino acids are eluted prior to the L-α-amino acids (reversed elution order for proline), show excellent separation factors α.
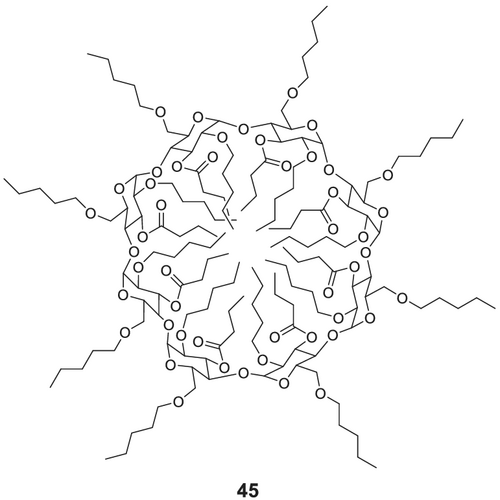
Octakis(3-O-butyryl-2,6-di-O-pentyl)-γ-CD 45 is synthesized (Scheme 11) from dry γ-CD 46 by pentylation of the O-2 and O-6 positions using n-pentyl bromide and powdered NaOH in anhydrous DMSO. To improve the overall yield, the base and alkylation reagent is again added after 2 days. Stirring for 5 days at room temperature followed by flash column chromatography yields the octakis(2,6-di-O-pentyl)-γ-CD 47. In a consecutive step, the butyrylation is achieved with butyric acid anhydride and DMAP under reflux in dichloromethane (DCM) after 10 days.
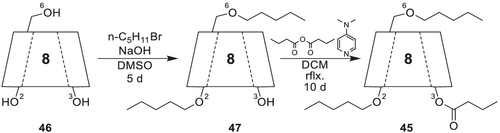
Octakis(3-O-butyryl-2,6-di-O-pentyl)-γ-CD 45 was originally developed for use as CSP in pyrex glass columns. For use in fused-silica columns, dilution of the selector (5 to 30% w,w) in the polysiloxane PS 255 (copolymer of dimethylsiloxane and 1–3% methylvinylsiloxane) has proven to be favorable.
As already mentioned, N-trifluoroacetyl amino acid methyl esters are very well separated as summarized in Table 7, and therefore, octakis(3-O-butyryl-2,6-di-O-pentyl)-γ-CD represents a complementary CSP to Chirasil-Val.
| Compound | Separation temperature T (°C) | Separation factor α | First eluted |
|---|---|---|---|
| Alanine A | 130 | 1.100 | D |
| Valine V | 130 | 1.148 | D |
| Isoleucine I | 130 | 1.139 | D |
| Leucine L | 130 | 1.126 | D |
| Threonine T | 130 | 1.069 | D |
| Serine S | 130 | 1.153 | D |
| Proline P | 130 | 1.173 | L |
| Cysteine | 160 | 1.042 | D |
| Methionine M | 160 | 1.053 | D |
| Phenylalanine F | 170 | 1.038 | D |
| Ornithine Orn | 180 | 1.112 | D |
| Lysine K | 180 | 1.184 | D |
| Aspartic acid D | 160 | 1.163 | D |
| Glutamic acid E | 160 | 1.065 | D |
| Tryptophane W | 190 | 1.014 | D |
| Tyrosine Y | 175 | 1.024 | D |
| α-Aminobutyric acid | 150 | 1.107 | D |
- Note: Fifty meter Pyrex glass capillary column, 0.25 mm i.d., coated with octakis(3-O-butyryl-2,6-di-O-pentyl)-γ-CD) 45.171
Furthermore, octakis(3-O-butyryl-2,6-di-O-pentyl)-γ-CD 45 shows also excellent separation factors for hydroxy carboxylic acids, derivatized as O-trifluoroacetyl/methyl esters, and chiral alcohols, derivatized as O-trifluoroacetyl esters (Table 8).
| Compound | Separation temperature T (°C) | Separation factor α | First eluted |
|---|---|---|---|
| Lactic acid | 70 | 1.320 | |
| 2-Hydroxybutyric acid | 100 | 1.065 | |
| 3-Hydroxybutyric acid | 80 | 1.926 | |
| 2-Hydroxyisovaleric acid | 100 | 1.024 | |
| 2-Hydroxycaproic acid | 100 | 1.125 | |
| 2-Hydroxyoctanoic acid | 100 | 1.121 | |
| 2-Hydroxydodecanoic acid | 150 | 1.021 | |
| Tartaric acid | 110 | 1.081 | D |
| Malic acid | 130 | 1.148 | D |
| Mandelic acid | 140 | 1.090 | D |
| 2-Methyl-1-penten-3-ol | 55 | 1,047 | |
| 1-Octen-3-ol | 75 | 1.053 | |
| 2-Octanol | 60 | 1.021 | |
| Myrtenol | 90 | 1.025 | |
| Borneol | 90 | 1.031 | |
| β-Citronellol | 90 | 1.014 | + |
- Note: Fifty meter Pyrex glass capillary column, 0.25 mm i.d., coated with octakis(3-O-butyryl-2,6-di-O-pentyl)-γ-CD 45.171
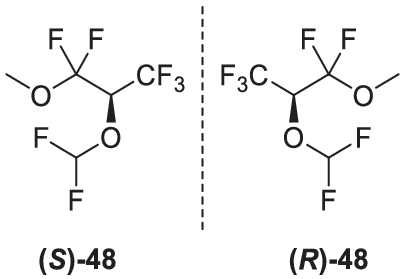
An exceptionally high separation factor was observed for the degradation product of the inhalational anesthetic sevoflurane (2-[fluoromethoxy]-1,1,1,3,3,3-hexafluoropropane),169, 171 compound B (2-[fluoromethoxy]-3-methoxy-1,1,1,3,3-pentafluoropropane) 48 (Figure 16), which forms under elimination and methanol addition. A separation factor α of 10.6 at 26°C has been reported for octakis(3-O-butyryl-2,6-di-O-pentyl)-γ-CD dissolved in PS 255.172
4.2.9 Hexakis/Heptakis/Octakis(2,6-di-O-alkyl-3-O-trifluoroacetyl)-α/β/γ-cyclodextrins
The first 2,6-dialkyl-3-trifluoroacetyl functionalized CD was reported by Nowotny et al.173 and Schurig et al.,174 who prepared and investigated the properties of heptakis(2,6-di-O-methyl-3-O-trifluoroacetyl)-β-CD 49 (Figure 17). The identically functionalized α- and γ-CDs were introduced by the Bicchi group.175-177 Li et al.178 synthesized 2,6-di-O-pentyl-3-O-trifluoroacetyl functionalized α-, β- and γ-CDs (commercially available as, e.g., Astec CHIRALDEX A-TA 50, Astec CHIRALDEX B-TA 51, Astec CHIRALDEX C-TA 52).
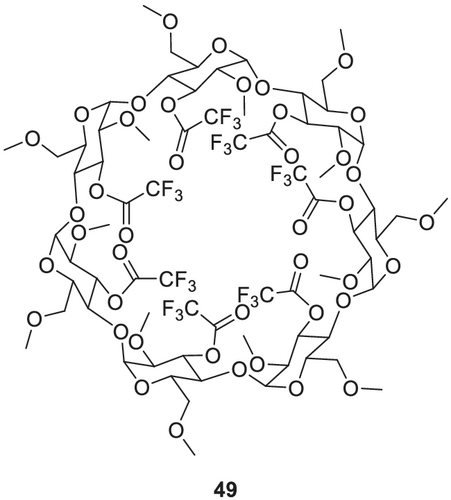
Heptakis(2,6-di-O-methyl-3-O-trifluoroacetyl)-β-CD 49 is prepared by adding BaO and Ba (OH)2 to a solution of β-CD in a DMSO/dimethyl formamide (DMF) mixture (Scheme 12).175 The dimethylated CD is obtained after 2 days of stirring. Reacting the dimethylated CD with trifluoroacetic anhydride and sodium trifluoroacetate for 12 h and subsequent addition of tetrachloromethane (CCl4) and refluxing for another 6 h yields the 2,6-di-O-methyl-3-O-trifluoroacetyl functionalized β-CD. Bicchi et al.177 slightly varied this procedure by employing methyl iodide instead of dimethyl sulfate and shortened the reaction time to 15 h. In the second step, they used pyridine instead of sodium trifluoroacetate and decreased the reaction time to 14 h.
The 2,6-di-O-pentylated CD derivatives are prepared by stirring a mixture of the dry CD and an excess of n-pentyl bromide with NaOH in DMSO for 6 h at 60°C (Scheme 13).179 The dipentylated CD derivative is subsequently refluxed with trifluoroacetic anhydride in THF for 2 h to yield the 2,6-di-O-pentyl-3-O-trifluoroacetyl functionalized CDs.
Bicchi and Schurig diluted the 2,6-di-O-methyl-3-O-trifluoroacetyl-CDs in polysiloxane OV-1701 and successfully applied the CSP in the separation of racemic compounds such as lactones, dioxolanes, spiroketals and terpenes, often showing complementary selectivity to their permethylated analogs.175-178, 180 However, they also observed faster degradation of column performance for 2,6-di-O-methyl-3-O-trifluoroacetyl functionalized α- and β-CDs compared to their permethylated counterparts.180 Li et al.178 applied the 2,6-di-O-pentyl-3-O-trifluoroacetyl-α/β/γ-CD selectors in the separation of more than 150 racemic compounds. Selected results of the screening are shown in Table 9.
| Compound | Separation temperature T (°C) | Separation factor α | CD |
|---|---|---|---|
| 2-Butanol | 40 | 1.22 | β |
| 40 | 1.16 | γ | |
| 2-Octanol | 70 | 1.06 | β |
| 40 | 1.15 | γ | |
| trans-1,2-Cyclohexane-diol | 70 | 1.58 | γ |
| 2-Amino-1-propanol | 100 | 1.07 | α |
| 110 | 1.16 | β | |
| 100 | 1.99 | γ | |
| Leucinol | 120 | 1.06 | α |
| 110 | 1.14 | β | |
| 2-Aminobutane | 80 | 1.04 | γ |
| Lactic acid | 50 | 1.47 | γ |
| Tartaric acid | 90 | 1.04 | β |
| Mandelic acid | 110 | 1.04 | β |
| 2-Bromo-1-chloro-propane | 40 | 1.06 | α |
| 40 | 1.12 | β | |
| 30 | 1.05 | γ | |
| Phenyloxirane | 80 | 1.01 | β |
| 80 | 1.57 | γ | |
| (E)-1,2-Diphenyloxirane | 140 | 1.02 | γ |
| β-Butyrolactone | 110 | 1.14 | α |
| 70 | 1.62 | β | |
| 80 | 1.20 | γ | |
| Isoborneol | 70 | 1.05 | γ |
| Carvone | 90 | 1.04 | α |
| 110 | 1.09 | β | |
| 100 | 1.01 | γ |
- Note: All amino and hydroxy functionalities were derivatized with trifluoroacetic anhydride. All carboxylic acid functionalities were derivatized as methyl esters. 10 m fused silica capillary column, 0.25 mm i.d., coated with hexakis/heptakis/octakis(2,6-di-O-pentyl-3-O-trifluoroacetyl)-α/β/γ-CD.179
The 2,6-di-O-pentyl-3-trifluoroacetyl functionalized CDs showed high separation ability for many compound classes, such as alcohols, amino alcohols, amines, diols, carboxylic acid esters, epoxides, and lactones. Especially, the γ-CD phase proved to be the most versatile CSP among the tested, separating 120 racemic compounds.
A concern when using trifluoroacetylated columns is their susceptibility towards hydrolysis, especially under higher separation temperatures, leading to reduced column lifetimes. To ensure longer lifetime, trifluoroacetyl modified columns have to be treated carefully with regard to solvents, water, and temperature exposure.179, 180 A reactivation is possible by on-column reaction with trifluoroacetic anhydride.
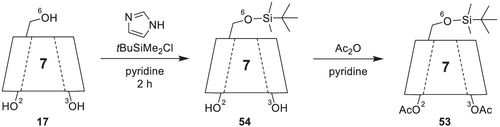
4.2.10 Heptakis(2,3-di-O-acetyl-6-O-tert-butyldimethylsilyl)-β-cyclodextrin (DIAC-6-TBDMS-β-CD)
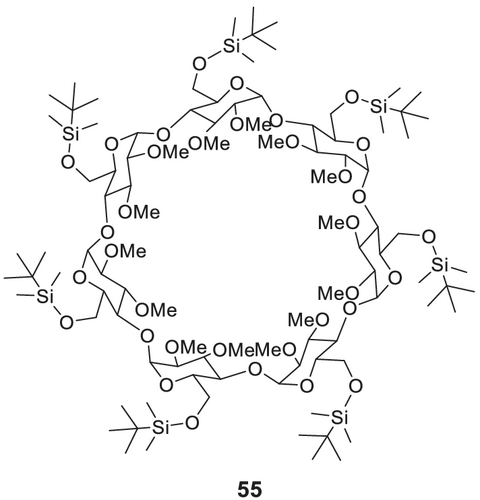
Per-O-alkylated and dialkylated/monoacylated derivatives of CDs are versatile chiral stationary phases for the separation of chiral flavor compounds.141 The use of 6-tert-butyldimethylsilylated and 2,3-diacylated (cf. Figure 18)180 53 (commercially available as, e.g., Supelco beta-DEX 225; MEGA-DEX DAC-Beta) or 2,3-dialkylated (cf. Figure 19) 55 β-CD derivatives was reported by Dietrich et al.179 for the simultaneous stereodifferentiation of a wide range of chiral flavor and fragrance181 compounds with different functionalities.
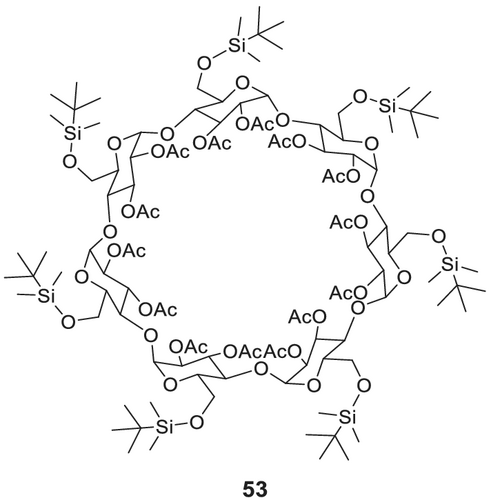
6-TBDMS-β-CD is synthesized (Scheme 14) by silylation of β-CD in O-6 position with imidazole and tert-butyldimethylsilyl chloride in pyridine. The mixture is stirred for 2 h, and the product is isolated by column chromatography or by recrystallization from hot n-heptane. In a second step, diacetylation is performed with acetic anhydride in pyridine, followed by the isolation of DIAC-6-TBDMS-β-CD via column chromatography as white crystals.
Lactones, which are the intramolecular esters of the corresponding hydroxy fatty acids and chiral 2-alkylalkanols (e.g., 2-methylbutanol and 1-octen-3-ol), can be separated using DIAC-6-TBDMS-β-CD 53 dissolved in the polysiloxane PS086 (50%/50%). This CD derivative was also applied to the chiral differentiation of rose oxides, such as citronellol, linalool, and carvone.
4.2.11 Heptakis(2,3-di-O-methyl-6-O-tert-butyldimethylsilyl)-β-cyclodextrin (DIME-6-TBDMS-β-CD)
In addition to heptakis(2,3-di-O-acetyl-6-O-tert-butyldimethylsilyl)-β-CD 53, heptakis(2,3-di-O-methyl-6-O-tert-butyldimethyl-silyl)-β-CD 55 (cf. Figure 19; commercially available as, e.g., CycloSil-B; Astec CHIRALDEX B-DM; MEGA-DEX DMT-Beta; Supelco beta-DEX 325)182 was also investigated as chiral stationary phase. Their selectivity is comparable, but the methylated derivative exhibits better solubility.
As already mentioned, 6-TBDMS-β-CD 54 is synthesized (Scheme 15) by silylation of β-CD 17 in O-6 position with imidazole and tert-butyldimethylsilyl chloride in pyridine. In a consecutive step, esterification of 6-TBDMS-β-CD is carried out in O-2 and O-3 positions with sodium hydride and methyl iodide in DMSO and dioxane. The reaction is completed after stirring over night at room temperature, followed by isolation of DIME-6-TBDMS-β-CD via column chromatography as white crystals.
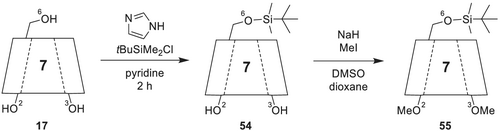
In comparison with permethyl-β-CD 16, the separation of various enantiomers (e.g., filbertone, β-pinene, borneol, methyl jasmonate, 3-mercaptohexanol, and tetramezine182) was improved using DIME-6-TBDMS-β-CD 55 dissolved in the polysiloxane PS086 (50%/50%).183 In addition, it is possible to separate cis- and trans-nerolidol at 80°C. Furthermore, alkyl-branched acids and their corresponding esters, which are important flavors, can be separated using DIME-6-TBDMS-β-CD 55 as CSP.184 Another versatile derivative is heptakis(6-O-tert-butyldimethylsilyl-2,3-di-O-ethyl)-β-CD,185, 186 which can be also used as a diluted selector dissolved in PS086 (50%/50%). This CSP allows to separate for example diaziridine derivatives with small alkyl substituents at the stereogenic nitrogen atoms.186, 187
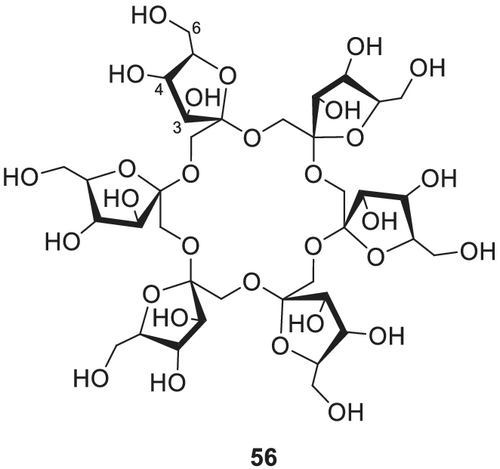
4.2.12 Cyclofructans
Cyclofructans (CFs) are cyclic carbohydrates consisting of 6 (α-CF, Cycloinulohexaose, Figure 20) 56, 7 (β-CF, Cycloinuloheptaose) 57 or 8 (γ-CF, Cycloinulooctaose) 58 fructose units that are connected by β-(2 → 1)-glycosidic bonds. Because of their linkage, CFs form a crown ether core in their center, which can be made accessible for substrate binding by derivatization of the 3-hydroxy groups. The application of derivatized CFs as chiral stationary phases in GC was pioneered by Zhang et al.,188 who employed permethylated α-CF, permethylated β-CF and 4,6-di-O-pentyl α-CF.
The permethylated CFs are synthesized by adding a solution of the CF in DMSO to a solution of NaH in DMSO and subsequently adding CH3I to the mixture. 4,6-di-O-pentyl α-CF is prepared analogous to its CD counterpart by reacting α-CF with an excess of 5-bromopentane and NaOH in DMSO.179
The permethylated CF selectors exhibit enantioselectivity towards alcohols, esters, β-lactams, and derivatized amino acids. A selection of compounds that were separated on permethylated α-CF is given in Table 10.
| Compound | Separation temperature T (°C) | Separation factor α |
|---|---|---|
| Alanine A | 50 | 1.03 |
| Valine V | 60 | 1.03 |
| Isoleucine I | 50 | 1.13 |
| Leucine L | 60 | 1.05 |
| Methionine M | 100 | 1.02 |
| Phenylalanine F | 100 | 1.01 |
| Aspartic acid D | 70 | 1.02 |
| (±)-α-(Trifluoromethyl)-benzylalcohol | 50 | 1.05 |
| Tartaric acid | 65 | 1.03 |
| cis-7-Azabicyclo[4.2.0]-oct-3-en-8-one | 100 | 1.05 |
- Note: All hydroxy and amino groups were trifluoroacetylated and the carboxyl groups methylated. Ten meter fused silica capillary column, coated with permethylated α-CF dissolved in polysiloxane OV-1701 (15% by weight).189
While more amino acids could be separated on permethylated α-CF, separation of β-lactams was improved on permethylated β-CF. For most compounds, separation factors were diminished on 4,6-di-O-pentyl α-CF, indicating a negative influence of the free 3-hydroxy groups on chiral recognition. Compared to their CD analogs, separation factors are lower on CFs. This can be attributed to a lack of inclusion interaction in CF selectors.
In a further report, Zhang and Armstrong190 prepared the 3-trifluoroacetyl derivative and the 3-propionyl derivative of 4,6-di-O-pentyl α-CF. The former is synthesized by repeatedly adding trifluoroacetic anhydride to a solution of 4,6-di-O-pentyl α-CF in anhydrous THF and refluxing the mixture, while the latter is synthesized by adding propionic anhydride to a solution of 4,6-di-O-pentyl α-CF in anhydrous THF and refluxing the mixture.
Both chiral stationary phases were investigated for the separation of derivatized amino acids, amino alcohols, amines, alcohols, tartrates, and lactones where they generally showed similar separation factors. Selected separation factors for the 3-trifluoroacetyl derivative (4,6-di-O-pentyl-3-O-trifluoroacetyl α-CF) are shown in Table 11.
| Compound | Separation temperature T (°C) | Separation factor α |
|---|---|---|
| Alanine A | 40 | 1.03 |
| Valine V | 50 | 1.03 |
| Isoleucine I | 100 | 1.06 |
| Leucine L | 70 | 1.03 |
| Serine S | 60 | 1.01 |
| Asparagine N | 80 | 1.01 |
| Proline P | 70 | 1.01 |
| Methionine M | 80 | 1.02 |
| Aspartic acid D | 80 | 1.01 |
| Glutamic acid E | 100 | 1.01 |
| α-Aminobutyric acid | 50 | 1.03 |
| 2-Amino-1-propanol | 45 | 1.01 |
| (±)-trans-1,2-Diamino-cyclohexane | 115 | 1.04 |
| (±)-trans-1,2-Cyclo-hexanediol | 40 | 1.01 |
| (±)-α-(Trifluoromethyl)-benzylalcohol | 70 | 1.03 |
| Tartaric acid | 60 | 1.01 |
| γ-Valerolactone | 45 | 1.01 |
- Note: All hydroxy and amino groups were trifluoroacetylated and the carboxyl groups methylated. Twenty meter salt treated fused silica capillary column, coated with 4,6-di-O-pentyl-3-O-trifluoroacetyl α-CF.191
Through thermodynamic analysis, the absence of an inclusion complex for CFs could be further supported. Instead, CF-analyte interactions most likely resemble a looser external association of several analyte molecules per CF selector.191
4.3 Chiral stationary phases with metal complexes
Chiral metal-chelates are a privileged class of highly versatile chiral selectors and were successfully applied in enantioselective complexation gas chromatography (GC). Chiral transition metal and rare earth metal complexes, such as metal 3-(trifluoroacetyl)-(1R)-camphorates,189, 191 were utilized as CSP because of their extraordinarily high enantioselectivities in separating chiral compounds. Later, Mn (II),192 Co (II),127 and Ni (II) β-diketonate complexes were introduced.
The versatility of alkanoyl-camphorate metal complexes as chiral selectors in enantioselective chromatography, as chiral shift reagents in NMR spectroscopy and as catalysts in asymmetric syntheses emphasize the importance to make these diketonate ligands easily accessible and to improve chemical properties, for example, decreasing the high volatility, which typically limits the applicable temperature range in enantioselective complexation GC. Higher temperatures lead to leaching of the chiral selector, which decreases the separation efficiency and limits the overall lifetime. To improve the thermal stability and to decrease leaching, the chiral metal-containing selector can be bonded to hydridomethyldimethylpolysiloxane by Pt-catalyzed hydrosilylation, a strategy commonly used to immobilize catalysts, as demonstrated by Schurig et al.147 This results in stationary phases with improved thermal stabilities and decreased column bleeding, which is of importance for mass spectrometric detection. The pivotal step to prepare this stationary phase was the synthesis of 10-methylenecamphor 59 from camphor-10-sulfonic acid chloride 60 and diazomethane (Scheme 16).
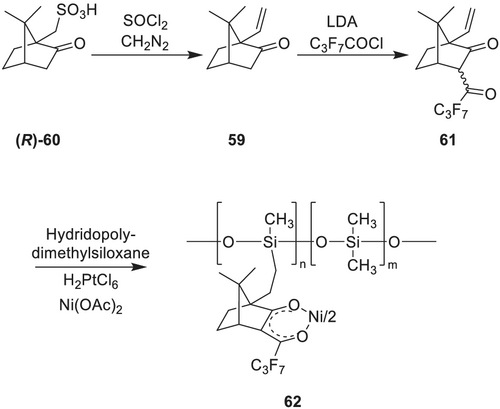
Another strategy is to introduce a hydroxy or thiol group at C-10 in the camphor moiety and achieve an immobilization via the formation of ether or thioether linkers with variable linker size.193
Starting from commercially available, enantiomerically pure (1S)-(+)-camphorsulfonic acid (S)-60 yields (1S,4R)-10-iodocamphor 63 in quantitative yields (>98%) using iodine and triphenylphosphine (Scheme 17). Purification by sublimation of (1S,4R)-10-iodocamphor allows to prepare large quantities. Reaction with excess potassium acetate and acetic acid under molten conditions (>175°C) results in the corresponding 10-acetatocamphor derivative 64 in quantitative yields (>97%). (1R,4R)-10-hydroxycamphor 65 is then prepared from the acetate by reaction in 10% (w,w) methanolic solution of potassium hydroxide under reflux conditions. Ether synthesis to yield (1R,4R)-10-allyloxycamphor 66 is performed using 10-hydroxycamphor and allylbromide under Williamson's ether synthesis conditions with NaH in THF.
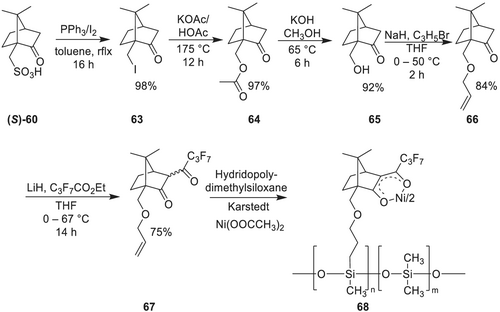
Lithium hydride is the base of choice to achieve the C-fluoroacylation of the camphor moiety 66. Addition of the fluorinated alkyl esters, instead of acyl chlorides, gives the desired product in good yield and purity.
For immobilization, hydridomethyldimethylpolysiloxanes (HMPS, Mw ~ 3,000 g/mol) with varying content of free silane groups can be used. Immobilization is achieved by Pt-catalyzed hydrosilylation reaction of 10-allyloxycamphor and HMPS using Pt-divinyltetramethyldisiloxane (Karstedt's catalyst) in anhydrous toluene under ultrasonification for 10 h at elevated temperatures. Metal incorporation can be achieved by reaction with nickel (ii) acetate tetrahydrate dissolved in methanol in a two-phase mixture. This mixture becomes miscible at elevated temperatures and re-separates upon cooling and purification, resulting in nickel (ii) bis([1R,4S]-3-heptafluorobutanoyl-10-propylenoxycamphorates) 68 immobilized on polysiloxane as pale green to green oils.
These CSPs show high separation factors α for a broad range of compounds, in particular for chiral oxirane 69–82 derivatives like (R,S)-methyloxirane (α = 1.27) (Table 12).
Besides polysiloxanes and polyethylene glycols, room temperature ionic liquids (ILs), which are low melting salts (<100°C), were developed as a new class of solvent matrices. ILs have a high viscosity, good wettability, and high thermal stability and exhibit dual nature; that is, they show both polar and nonpolar behavior. There are two different ways to use ILs in chiral separation: (1) Achiral ionic liquids can be used as solvents for chiral selectors, or (2) the ionic liquid can be chiral and coated on the capillary column.129, 194 Chiral IL stationary phases in GC were investigated for the enantiomeric separation of alcohols, diols, sulfoxides, epoxides, and acetylated amines.195, 196Because of the exceptional polarity and low volatility properties, and as demonstrated by the broad applicability in chiral GC separations, ionic liquids are promising (chiral) solvents and potential backbones to immobilize chiral selectors.
5 CONCLUSION
The here discussed chiral stationary phases ranging from diamide selectors, CDs, to chiral metal complexes enable enantiomer separation of a broad range of compounds and therefore constitute an indispensable tool for the characterization and quantification of enantiomeric ratios in asymmetric synthesis, catalysis, medicinal chemistry, chemical biology, and drug research.
ACKNOWLEDGMENTS
Gloria Betzenbichler, Laura Huber, Sabrina Kräh, Marie-Louise K. Morkos, and Alexander F. Siegle contributed equally to this work.