Trustworthiness assessment of published clinical trials: Literature review of domains and questions
Abstract
Background
Historically, peer reviewing has focused on the importance of research questions/hypotheses, appropriateness of research methods, risk of bias, and quality of writing. Until recently, the issues related to trustworthiness—including but not limited to plagiarism and fraud—have been largely neglected because of lack of awareness and lack of adequate tools/training. We set out to identify all relevant papers that have tackled the issue of trustworthiness assessment to identify key domains that have been suggested as an integral part of any such assessment.
Methods
We searched the literature for publications of tools, checklists, or methods used or proposed for the assessment of trustworthiness of randomized trials. Data items (questions) were extracted from the included publications and transcribed on Excel including the assessment domain. Both authors then independently recategorised each data item in five domains (governance, plausibility, plagiarism, reporting, and statistics).
Results
From the 41 publications we extracted a total of 284 questions and framed 77 summary questions grouped in five domains: governance (13 questions), plausibility (17 questions), plagiarism (4 questions), reporting (29 questions), and statistics (14 questions).
Conclusion
The proposed menu of domains and questions should encourage peer reviewers, editors, systematic reviewers and developers of guidelines to engage in a more formal trustworthiness assessment. Methodologists should aim to identify the domains and questions that should be considered mandatory, those that are optional depending on the resources available, and those that could be discarded because of lack of discriminatory power.
1 INTRODUCTION
Historically, peer reviewing has focused on the importance of research questions/hypotheses, appropriateness of research methods, risk of bias, and quality of writing. Until recently, the issues related to trustworthiness, including but not limited to plagiarism and fraud, have been largely neglected because of lack of awareness and lack of adequate tools/training.
Labeling any published work as untrustworthy is fraught with difficulties as there is very little incentive for peer reviewers, editors, publishers, and institutions to put their head above the parapet [1, 2]. The evidence points to an unacceptable reluctance of journals and publishers to engage in this agenda [3, 4] even though scientific fraud, including the use of paper mills and AI-generated papers, is now being highlighted as a critical credibility issue reported by major print media as well as scientific journals [5-9]. The lack of appropriate tools has been cited as a major obstacle [10], despite the fact that a relatively large number of papers have been published on this topic.
We aimed to counter obstacles with a literature overview of all relevant papers that have tackled the issue of trustworthiness assessment and of all key domains and questions suggested as an integral part of any such trustworthiness assessment.
To facilitate the reviewing process, we focused on the importance of clinical trial findings for health and treatment decisions. Therefore, for the purposes of this study we have defined trustworthiness as study data being reliable because of the integrity of both the research process and the intentions of those involved. Armond and colleagues have recently published an important overview of how scholarly literature has thought about research integrity definitions and challenges [11]. They define research integrity and its domains as “the conduct of the research process ethically, with honesty, robustness, and transparency when proposing, conducting, evaluating, and reporting research findings. It involves compliance with rules, regulations, and guidelines, as well as widely accepted professional codes and norms” [11]. They describe how responsible conduct of research is at the high end of a research integrity continuum that descends through questionable or detrimental practices, such as sloppy data management, poor mentorship and biased reporting, to outright research fraud—fabrication, falsification, and plagiarism—at its lowest. This definition chimes with the 2023 Cairo Consensus Statement on Clinical Trial Integrity, which emphasizes integrity throughout the research life cycle [12].
- 1.
Given the continuum of research integrity as described above, we recognize that assessing trustworthiness may, though not intentionally, overlap with the assessment of bias, which is usually already covered by other tools (for instance, the Cochrane Risk of Bias tool [13]).
- 2.
The focus on data means that the trustworthiness tools reviewed here are likely to have a slightly narrower focus than that of research integrity (research integrity assessment might look at, for instance, whether a research question is ethical).
- 3.
The focus on intention differs from traditional assessments of bias, as up until recently it has been generally assumed that researchers always act with the best of intentions, and the possibility of research fraud has not been considered.
This paper adds to the literature because it sorts tools that make trustworthiness assessments into clear conceptual domains, summed up with short lists of user-friendly questions. We have not evaluated domains and questions posed for validity, effectiveness, or ease of use, or attempted to provide another trustworthiness tool, as this work is being done elsewhere, for instance by Cochrane [14]. Rather, we hope that this paper will serve as a useful first call for those wanting to access the literature and a useful conceptual clarification and aide-memoire for those already involved in creating and using trustworthiness tools. We hope that it will also encourage the use of trustworthiness assessment as an integral part of the peer review process.
2 METHODS
To identify common domains/questions, we searched the literature for publications of tools, checklists or methods used or proposed for the assessment of trustworthiness of randomized trials, irrespective of how many items they had. The following databases were searched using search strings with the terms “tool,” “assessment,” “trustworthiness,” “randomized controlled trial,” “plagiarism,” “fabrication,” and “research integrity.”
-
Medline via PubMed
-
Scopus
-
Web of Science
-
Google Scholar.
We included publications already known to the authors at the time of the search and also screened the reference lists of all included publications for additional references. Our search was restricted to publications in English language as we did not have resources for full translations from other languages. The date of the last search was January 16, 2024.
2.1 Selection of studies and data extraction
-
Governance: questions covering the initial set-up and overview of the research process, related to decision-making and accountability processes.
-
Plausibility: questions to do with whether statements within published reports are possible, plausible, and make sense.
-
Statistics: further independent verification of the data from the published report using additional statistical testing to check for accuracy and plausibility.
-
Other: any item or question that could not be classified in these three domains
Both authors then independently assessed the objectivity of each question. An item/question was categorized as “objective” if it could be answered with a clear YES or NO answer backed with available written evidence or “subjective” if an answer would be a matter of opinion.
-
Plagiarism: to do with unjustifiable copying
-
Reporting: questions of missing/insufficient data or methodology or inappropriate use of AI software/IT technology.
Both authors agreed on the final allocation of the questions to each domain. J. W. then reorganized the Excel file so that each question was allocated to its new domain. Both authors then worked together to create and sort user-friendly questions summarizing the questions for each domain.
3 RESULTS
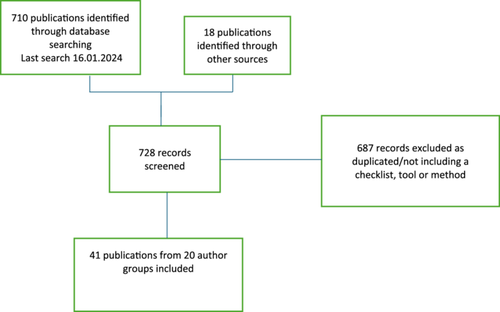
Our initial search yielded 710 publications with an additional 18 that were added either from publications already known to the authors or from reference lists (Figure 1). Once 687 records were excluded, we reviewed 41 publications comprising 40 publications of tools/checklists/methods and one tool/checklist being developed and published in protocol form at the time of the literature search (Figure 1).
From the 41 publications we extracted a total of 284 questions and listed them according to 20 author groups (Table 1). Some of these questions were duplicates as they have been published at least twice (for instance, all questions in REAPPRAISED have been republished in INSPECT-SR). The number of identified questions per publication ranged from 1 to 70, of which 206 (73%) had been allocated domains in their original publications. For the 206 questions with domains already allocated, we assigned 14 to the original domains; 26 questions had negligible domain change (e.g., “governance” instead of “research governance” and “governance statements,” or “plausibility” instead of “plausibility of intervention”); and 166 were assigned to different domain names. For the 78 questions with no domains, J. W. and Z. A. independently assigned the same domain for 50 questions, and for the rest the final domain was assigned after discussion.
| Checklist/author/author group; number of publications; number of questions | Question | Original domain (if given) | Agreed new domain |
|---|---|---|---|
Al Marzouki et al. [20] 1 publication 1 question |
Are the statistics feasible? | Statistics | |
Anderson et al. [21] 1 publication 5 questions |
Is the trial prospectively registered and numbered? | Governance | |
| Is there an ethics approval statement with a named committee? | Governance | ||
| Is there a statement on informed consent? | Governance | ||
| Does the trial adhere to CONSORT guidelines? | Governance | ||
| Is there a data sharing statement? | Governance | ||
Berghella et al. [22] 1 publication 6 questions |
Is there prospective registration in a World Health Organization International Clinical Trials Registry Platform with date and registry number provided? | Governance | |
| Is there a statement of ethical approval with the name of the approving committee? | Governance | ||
| Is there a statement of informed consent? | Governance | ||
| Is there a statement of adherence to CONSORT guidelines or a CONSORT checklist? | Governance | ||
| Is there an agreement to provide anonymized data to the journal if requested? | Governance | ||
| Is there a data availability statement? | Governance | ||
| Bolland group [23-27] | |||
5 publications 9 questions |
Do the randomization outcomes suggest unreliability of the RCT results? | Plausibility | |
| Does the distribution of participant withdrawals suggest integrity concerns? | Statistics | ||
| Do the distribution of baseline categorical variables differ from expected distributions? | Plausibility | ||
| Is there an alert on the article? | Governance | ||
| Are there identical summary statistics? | Statistics | Statistics | |
| Are there highly unusual P-value distributions in groups of RCTs that might raise or reinforce concerns about randomization and data integrity? | Statistics | Statistics | |
| As above, but allow for rounded summary statistics | Statistics | Statistics | |
| Are participant withdrawals unusually distributed? | Plausibility | Plausibility | |
| Are there unusual distributions of baseline categorical variables? | Plausibility | Plausibility | |
Byrne and Christopher [28] 1 publication 3 questions |
Is there a specific study hypothesis? | Reporting | |
| Is the experiment tailored to the hypothesis? | Reporting | ||
| Is the experiment and results fully described? | Plausibility | ||
| Carlisle group [14, 29-34] | |||
7 publications 9 questions |
Does statistical analysis suggest problematic data integrity? | Statistics | |
| Has false data already been noted with this author or institute? | Governance | ||
| Are there inconsistencies in registered protocols? | Governance | ||
| Is content copied from published papers? | Plagiarism | ||
| Are there unusually dissimilar or similar mean (SD) values for baseline variables? | Statistics | ||
| Are the results incredible? | Plausibility | ||
| Are calculations incorrect? | Statistics | ||
| With individual patient data only: are data duplicated in rows and columns of spreadsheets? | Plausibility | ||
| Is there evidence for duplication of figures? | Plagiarism | ||
Committee on Publication Ethics (COPE) guidelines [35] 1 website 1 question |
Does the study follow COPE guidelines? | Governance | |
| Cochrane 6 publications, listed separately as they include 3 major checklists | |||
Cochrane editorial policy Mann [36] Boughton et al. [37] 2 publications 5 questions |
Are there any post-publication amendments (expressions of concern, errata, corrigenda and s)? | Governance | |
| Is there possible duplication of text? | Plagiarism | ||
| Is there possible duplication of data? | Plagiarism | ||
| Is there implausible distribution of data? | Plausibility | ||
| Is there unlikely distribution of patients between groups? | Plausibility | ||
Cochrane Pregnancy and Childbirth Trustworthiness Screening Tool (CPC-TST) checklist Cochrane Pregnancy and Childbirth Editorial Board [38] 1 website file 11 questions |
Are there any retraction notices or expressions of concern listed on the Retraction Watch database relating to this study? | Research governance | Governance |
| Was the study prospectively registered (for those studies published after 2010)? If not, have the authors provided a plausible reason? | Research governance | Governance | |
| When requested, did the trial authors provide/share the protocol and/or ethics approval letter? | Research governance | Governance | |
| Did the trial authors engage in communication with Cochrane Pregnancy and Childbirth within the agreed timelines? | Research governance | Governance | |
| Did the trial authors provide IPD data upon request? If not, was there a plausible reason? | Research governance | Plausibility | |
| Do characteristics of the study participants appear too similar? | Baseline characteristics | Plausibility | |
| Are results implausible? | Feasibility | Plausibility | |
| In cases with (close to) zero losses to follow-up, is there a plausible explanation? | Feasibility | Plausibility | |
| Do the numbers randomized to each group suggest that adequate randomization methods were used? | Feasibility | Plausibility | |
| If only an abstract has been published, have the study authors confirmed in writing that the data has come from the final analysis and will not change? | Reporting | ||
| If there are concerns, does correspondence with the authors give adequate information or answers? | Reporting | ||
Weeks et al. [39] 1 publication 1 question |
As for CPC-TST plus: have you allowed for the age of the study?
|
Governance | |
Parker et al. [40] 26 questions |
Is there an alert on the article or other publications by the team? | Existing alerts | Governance |
| Is the journal identified as predatory, low quality, or vulnerable? | Journal details | Reporting | |
| Is ethics committee approval/protocol preregistration information present? | Ethics concerns | Governance | |
| Is the style of acknowledgment/funding statement plausible? | Ethics concerns | Governance | |
| How plausible is the transparency of study methods/results? | Study aim, method & style | Reporting | |
| How plausible are the study details? | Study aim, method & style | Reporting | |
| How plausible is the study wording (does it have very unusual wording?) | Study aim, method & style | Reporting | |
| Does the methods section have evidence of expert input? | Study aim, method & style | Reporting | |
| Is participant recruitment plausible? | Patient baseline data | Plausibility | |
| Are participant characteristics plausible? | Patient baseline data | Plausibility | |
| Does comparing the mean and standard deviation between participant groups show plausible results? | Patient baseline data | Statistics | |
| Are results plausible when reviewed in the context of similar studies or topics? | Results | Plausibility | |
| Review other publications by the author/s: are there other publications retracted/expression of concern, relevant post publication amendment/s, critical Retraction Watch/PubPeer comment/s, or exclusion/s from systematic reviews? | Review other publications by author/s | Governance | |
| Check author affiliations, publication record, and name against email address name | Author/s details | Reporting | |
| If necessary, ask authors for detail about study; is the answer plausible? | Author/s details | Governance | |
| Is ethics approval plausible? (read carefully, entering details into search engine; check protocol registers for dates and details; enter acknowledgment/funding statements into search engine). | Governance statements | Governance | |
| Is the study location plausible? | Study aim, method, style | Plausibility | |
| Is the context plausible? | Study aim, method, style | Plausibility | |
| Is the hypothesis plausible? | Study aim, method, style | Reporting | |
| Is the method plausible? | Study aim, method, style | Reporting | |
| Is the study free from mistakes? | Study aim, method, style | Plausibility | |
| Is the study free from signs of manipulation? (especially Western blots, immunohistochemistry, and flow cytometry - check images carefully – either self or ask a consulting image team) | Study aim, method, style | Plausibility | |
| Are reported patient characteristic figures medically plausible? | Patient baseline data | Plausibility | |
| Are reported patient characteristic figures statistically plausible? (e.g., check if reported % of participants with a given characteristic could match a whole number) | Patient baseline data | Statistics | |
| Does participant details in the published article cross-check against registered protocol and IPD? | Patient baseline data | Reporting | |
| Are the IPD plausible? | Patient baseline data | Statistics | |
“INSPECT-R” checklist (in development) 1 publication 70 questions Wilkinson et al. [14] |
1. Are the results substantially divergent from the results of multiple other studies in meta-analysis? | Inspecting the results in the paper | Plausibility |
| 2. Are subgroup means incompatible with those for the whole cohort? | Inspecting the results in the paper | Plausibility | |
| 3. Are numbers of participants correct and consistent throughout the publication? | Inspecting the results in the paper | Reporting | |
| 4. Are there any discrepancies between the values for percentage and absolute change? | Inspecting the results in the paper | Statistics | |
| 5. Are data simulated from reported summary statistics plausible? | Inspecting the results in the paper | Statistics | |
| 6. Are the means of integer data possible? | Inspecting the results in the paper | Plausibility | |
| Is the amount of missing data plausible? | Inspecting the results in the paper | Plausibility | |
| Are results internally consistent (e.g., are there more births than pregnancies)? | Inspecting the results in the paper | Plausibility | |
| Are the variances in biological variables surprisingly consistent over time? | Inspecting the results in the paper | Plausibility | |
| Are correct units reported? | Inspecting the results in the paper | Reporting | |
| Are the variances of integer data possible? | Inspecting the results in the paper | Plausibility | |
| Are differences in variances in baseline variables between randomized groups plausible (using summary data, e.g., F test, Bartlett)? | Inspecting the results in the paper | Statistics | |
| Are there any discrepancies between reported data and participant inclusion criteria? | Inspecting the results in the paper | Plausibility | |
| Are any outcome data, including estimated treatment effects, implausible? | Inspecting the results in the paper | Plausibility | |
| Are any baseline data implausible with respect to magnitude or variance? | Inspecting the results in the paper | Plausibility | |
| Are statistical test results compatible with reported data? | Inspecting the results in the paper | Statistics | |
| Are the reported summary data compatible with the reported range? | Inspecting the results in the paper | Plausibility | |
| Is there heterogeneity across studies in degree of imbalance in baseline characteristics (in meta-analysis)? | Inspecting the results in the paper | Plausibility | |
| Is the number of participant withdrawals compatible with the disease, age, and timeline? | Inspecting the results in the paper | Plausibility | |
| Are any of the baseline data excessively different between randomized groups? | Inspecting the results in the paper | Plausibility | |
| Are there any discrepancies between data reported in figures, tables, and text? | Inspecting the results in the paper | Reporting | |
| Are non-first digits compatible with a genuine measurement process? | Inspecting the results in the paper | Statistics | |
| Are coefficients of variation plausible? | Inspecting the results in the paper | Statistics | |
| Are the summary outcome data identical or nearly identical across study groups? | Inspecting the results in the paper | Plausibility | |
| Are any of the baseline data excessively similar between randomized groups? (including e.g., Stouffer-Fisher, method of A Barnett) | Inspecting the results in the paper | Statistics | |
| Are calculations of proportions and percentages correct? | Inspecting the results in the paper | Statistics | |
| Does consideration of other studies from members of the research team highlight causes for concern? | Inspecting the research team | Governance | |
| Are duplicate-reported data consistent between publications? | Inspecting the research team | Governance | |
| Are relevant methods consistent between publications? | Inspecting the research team | Governance | |
| Are contributorship statements complete? | Inspecting the research team | Governance | |
| Is any duplicate reporting acknowledged or explained? | Inspecting the research team | Governance | |
| Do all authors meet criteria for authorship? | Inspecting the research team | Governance | |
| Have other studies from the author team been retracted, or do they have expressions of concern, relevant post-publication amendment, or critical Retraction Watch or PubPeer comment? | Inspecting the research team | Governance | |
| Does the statistics methods section use generic language, suggesting lack of expert statistical input? | Inspecting the research team | Reporting | |
| Do any authors have a professorial title but no other publications on PubMed? | Inspecting the research team | Governance | |
| Is there evidence of duplication of figures? | Inspecting the research team | Plagiarism | |
| Is authorship of related papers consistent? | Inspecting the research team | Governance | |
| Have the data been published elsewhere by the research team? | Inspecting the research team | Governance | |
| Is the standard deviation of summary statistics in multiple studies by the same authors plausible (when compared to simulated or bootstrapped data)? | Inspecting the research team | Statistics | |
| Are the authors on staff of institutions they list? | Inspecting the research team | Governance | |
| Is the distribution of non-first digits in manuscripts from one author compatible with a genuine measurement process? | Inspecting the research team | Plausibility | |
| Can co-authors attest to the reliability of the paper? | Inspecting the research team | Governance | |
| Are contributorship statements present? | Inspecting the research team | Governance | |
| Is the volume of work reported by the research group plausible, including that indicated by concurrent studies from the same group? | Inspecting conduct, governance and transparency | Governance | |
| Is the reported staffing adequate for the study conduct as reported? | Inspecting conduct, governance and transparency | Plausibility | |
| Is the recruitment of participants plausible within the stated time frame for the research? | Inspecting conduct, governance and transparency | Plausibility | |
| Is the recruitment of participants plausible considering the epidemiology of the disease in the area of the study location? | Inspecting conduct, governance and transparency | Plausibility | |
| Is the interval between study completion and manuscript submission plausible? | Inspecting conduct, governance and transparency | Plausibility | |
| Could the study plausibly be completed as described? | Inspecting conduct, governance and transparency | Plausibility | |
| Are the study methods plausible, at the location specified? | Inspecting conduct, governance and transparency | Reporting | |
| Are the locations where the research took place specified, and is this information plausible? | Inspecting conduct, governance and transparency | Reporting | |
| Is a funding source reported? | Inspecting conduct, governance and transparency | Reporting | |
| Has the study been prospectively registered? | Inspecting conduct, governance and transparency | Governance | |
| Are details such as dates and study methods in the publication consistent with those in the registration documents? | Inspecting conduct, governance and transparency | governance | |
| Is there evidence that the work has been approved by a specific, recognized committee? (ethics) | Inspecting conduct, governance and transparency | Governance | |
| Are there any concerns about unethical practice? | Inspecting conduct, governance and transparency | Governance | |
| Is the grant funding number identical to the number in unrelated studies? | Inspecting conduct, governance and transparency | Governance | |
| Are the data publicly available? | Inspecting conduct, governance and transparency | Governance | |
| Do the authors agree to share individual participant data? | Inspecting conduct, governance and transparency | Governance | |
| Are additional patient data recorded in patient case records beyond what is reported in the paper? | Inspecting conduct, governance and transparency | Governance | |
| Do authors cooperate with requests for information? | Inspecting conduct, governance and transparency | Governance | |
| Do authors provide satisfactory responses to requests? | Inspecting conduct, governance and transparency | Governance | |
| Has the study been retracted or does it have an expression of concern, a relevant post-publication amendment, a critical Retraction Watch or PubPeer comment or has been previously excluded from a systematic review? | Inspecting text and publication details | Governance | |
| Is there evidence of copied work, such as duplicated or partially duplicated tables? | Inspecting text and publication details | Plagiarism | |
| Is there evidence of manipulation or duplication of images? | Inspecting text and publication details | Plagiarism | |
| Are there typographical errors? | Inspecting text and publication details | Reporting | |
| Was the study published in a journal from a list of predatory/low-quality journals? | Inspecting text and publication details | Governance | |
| Is there evidence of text reuse (cutting and pasting text between papers), including text that is inconsistent with the study? | Inspecting text and publication details | Plagiarism/fraud | |
| Is there evidence of automatically-generated text? | Inspecting text and publication details | Reporting | |
| Are the individual personal data plausible? (33 methods – see Appendix 1) | Inspecting individual personal data | Statistics | |
Cole 2015 [41] 1 publication 5 questions |
Are there factual discrepancies, e.g., abstract and text mutually contradictory? | Plausibility | |
| Are there impossible percentages? | Statistics | ||
| Are there impossible summary statistics? | Statistics | ||
| Are there arithmetical errors? | Statistics | ||
| Are there missed p values? | Statistics | ||
Damen et al. 2023 [42] 1 publication 1 question |
Are there indicators of questionable research practices? | Reporting | |
“REAPPRAISED” checklist Grey et al. [43] 1 publication 54 questions |
Are the locations where the research took place specified? | Research governance | Governance |
| Are the locations where the research took place plausible? | Plausibility | ||
| Is a funding source reported? | Research governance | Governance | |
| Has the study been registered? | Research governance | Governance | |
| Are details (such as dates and study methods) in the publication consistent with those in the registration documents? | Research governance | Governance | |
| Is there evidence that the work has been approved by a specific, recognized committee? | Ethics | Governance | |
| Are there any concerns about unethical practice? | Ethics | Governance | |
| Do all authors meet criteria for authorship? | Authorship | Governance | |
| Are contributorship statements present? | Authorship | Governance | |
| Are contributorship statements complete? | Authorship | Governance | |
| Is authorship of related papers consistent? | Authorship | Governance | |
| Can coauthors attest to the reliability of the paper? | Authorship | Governance | |
| Is the volume of work reported by research group plausible, including that indicated by concurrent studies from the same group? | Productivity | Plausibility | |
| Is the reported staffing adequate for the study conduct as reported? | Productivity | Plausibility | |
| Is there evidence of copied work? | Plagiarism | Plagiarism | |
| Is there evidence of text recycling (cutting and pasting text between papers), including text that is inconsistent with the study? | Plagiarism | Plagiarism | |
| Is the recruitment of participants plausible within the stated time frame for the research? | Research conduct | Plausibility | |
| Is the recruitment of participants plausible considering the epidemiology of the disease in the area of the study location? | Research conduct | Plausibility | |
| Is the number of participant withdrawals compatible with the disease, age and timeline? | Research conduct | Plausibility | |
| Is the number of participant deaths compatible with the disease, age and timeline? | Research conduct | Plausibility | |
| Is the interval between study completion and manuscript submission plausible? | Research conduct | Plausibility | |
| Could the study plausibly be completed as described? | Research conduct | Plausibility | |
| Are the study methods plausible at the location specified? | Analyses & methods | Plausibility | |
| Is there missing (numerical) data? | Analyses & methods | Statistics | |
| Is there inappropriate data handling? | Analyses & methods | Statistics | |
| Is there “p-hacking” (biased or selective analyses that promote fragile results)? | Analyses & methods | Statistics | |
| Is there other unacknowledged multiple statistical testing? | Analyses & methods | Statistics | |
| Is there outcome switching—that is, does the analysis and discussion focus on measures other than those specified in registered analysis plans? | Analyses & methods | Reporting | |
| Is there evidence of manipulation or duplication of images? | Image manipulation | Plagiarism | |
| 1. Are subgroup means compatible with those for the whole cohort? | Statistics & data | Statistics | |
| 2. Are the reported summary data compatible with the reported range? | Statistics & data | Statistics | |
| 3. Are the summary outcome data identical across study groups? | Statistics & data | Statistics | |
| 4. Are there any discrepancies between data reported in figures, tables and text? | Statistics & data | Reporting | |
| 5. Are statistical test results compatible with reported data? | Statistics & data | Statistics | |
| 1. Are any of the baseline data excessively similar or different between randomized groups? | Statistics & data | Statistics | |
| 2. Are any of the outcome data unexpected outliers? | Statistics & data | Statistics | |
| 3. Are the frequencies of the outcomes unusual? | Statistics & data | Statistics | |
| 4. Are any data outside the expected range for sex, age or disease? | Statistics & data | Plausibility | |
| 5. Are there any discrepancies between the values for percentage and absolute change? | Statistics & data | Statistics | |
| 6. Are there any discrepancies between reported data and participant inclusion criteria? | Statistics & data | Plausibility | |
| 7. Are the variances in biological variables surprisingly consistent over time? | Statistics & data | Plausibility | |
| 1. Are correct units reported? | Errors | Reporting | |
| 2. Are numbers of participants correct and consistent throughout the publication? | Errors | Statistics | |
| 3. Are calculations of proportions and percentages correct? | Errors | Statistics | |
| 4. Are results internally consistent? | Errors | Reporting | |
| 5. Are the results of statistical testing internally consistent and plausible? | Errors | Statistics | |
| 6. Are other data errors present? | Errors | Statistics | |
| 7. Are there typographical errors? | Errors | Reporting | |
| Have the data been published elsewhere? | Data duplication & reporting | Reporting | |
| Is any duplicate reporting acknowledged or explained? | Data duplication & reporting | Reporting | |
| How many data are duplicate reported? | Data duplication & reporting | Reporting | |
| Are duplicate-reported data consistent between publications? | Data duplication & reporting | Reporting | |
| Are relevant methods consistent between publications? | Data duplication & reporting | Reporting | |
| Is there evidence of duplication of figures? | Data duplication & reporting | Reporting | |
| Hartgerink [44, 45] | |||
2 publications 3 questions |
Is there evidence of manipulation of data, e.g., p-hacking? | Statistics | |
| Is there evidence of fabricated data? | Plausibility | ||
| Do statistical tools suggest fabricated data? | Statistics | ||
Hill et al. [46] 1 publication 4 questions |
Does the χ2 test on baseline characteristics suggest an ineffective randomization process? | Statistics | |
| Were patients randomized into treatment arms on similar dates? | Plausibility | ||
| Was recruitment to treatment arms balanced at each investigation center? | Plausibility | ||
| (IPD) Is there any evidence of duplicate participants, unexpected homogeneity or unexpected heterogeneity? | Statistics | ||
Hodgson et al. [47] 1 publication 12 questions |
Does prospective registration accurately describe the trial? | Governance | Governance |
| Does published protocol accurately describe the trial (e.g., primary outcome unchanged)? | Governance | Governance | |
| Is the human research ethics committee approval clear? | Governance | Governance | |
| Is there clear allocation concealment? | Methods | Reporting | |
| Are the methods appropriate for the study design? | Methods | Reporting | |
| Is there a realistic timeframe, including follow-up? | Methods | Plausibility | |
| Are the baseline characteristics well described and plausible? | Methods | Plausibility | |
| Is there a realistic dropout rate? | Methods | Plausibility | |
| Are the effect sizes realistic compared to other RCTs on the same topic? | Methods | Plausibility | |
| Are the authors listed on an extraction database? | Author group | Governance | |
| Is there a low ratio of authors to study size? | Author group | Plausibility | |
| Is there a large number of RCTs published in a short time frame from the same author group? | Author group | Plausibility | |
Hsieh et al. [48] 1 publication 1 question |
Does the protocol paper report show adequate trial monitoring (e.g., follow SPIRIT guidelines)? | Governance | |
Hüllemann et al [49] 1 publication 1 question |
Is there an unusual distribution of leading digits (Benford's Law)? | Statistics | |
Lu et al. 2006 [50] 1 publication 1 question |
Using adaptive Benford's Law, are there indicators of anomalous behavior that are strong indicators of fraud? | Statistics | |
Trustworthiness in Randomized Controlled Trials (TRACT) checklist Mol et al. [51] 6 publications 23 questions |
Is there no/retrospective registration of RCTs (after 2010)? | Governance | Governance |
| Is there a discrepancy of >15% between the intended sample size in the trial registration compared to the actual sample size achieved in the RCT? | Governance | Governance | |
| Is there no/only vague description of research ethics? | Governance | Governance | |
| Are there other concerns regarding ethics? | Governance | Governance | |
| Are there 3 or less authors, or a low author-to-study size ratio? (subjective, based on field of study, author/group, and timeframe) | Author group | Governance | |
| Have other studies of any of the authors have been retracted, not on their request? | Author group | Governance | |
| Does Retraction Watch show anything problematic about the author? | Author group | Governance | |
| Are there a large number of RCTs in a short time frame by one author/one institute? | Author group | Plausibility | |
| Is the description of allocation concealment sufficient? | Plausibility of intervention | Reporting | |
| Is the description of allocation concealment plausible? | Plausibility of intervention | Plausibility | |
| Is there unnecessary or illogical description of methodological standards? | Plausibility of intervention | Reporting | |
| Is there a fast recruitment of participants within the study time (especially single center studies)? | Timeframe | Plausibility | |
| Is there a short or impossible time frame between ending recruitment, follow-up, and submission of paper? | Timeframe | Plausibility | |
| Are there zero participants lost to follow-up? | Dropout rates | Plausibility | |
| Are there no reasons mentioned for loss of follow-up, especially in cases of long follow-up and/or multiple cycles of or long-lasting interventions? | Dropout rates | Reporting | |
| Are there an ideal number of losses to follow-up, resulting in perfectly rounded numbers in each group (e.g., groups of 50 or 100)? | Dropout rates | Plausibility | |
| Are at least five characteristics presented? | Baseline characteristics | Reporting | |
| Are patient characteristics plausible, judging from common sense, the literature and local data? | Baseline characteristics | Plausibility | |
| Is there a perfect balance for multiple characteristics, or significant/large differences between characteristics? | Baseline characteristics | Plausibility | |
| Are important prognostic factors not reported as baseline characteristics? | Baseline characteristics | Reporting | |
| Is there an effect size that is much larger than in other RCTs regarding the same topic? (consider utilizing meta-analyses if available) | Outcomes | Plausibility | |
| Is there conflicting information between outcomes? | Outcomes | Plausibility | |
| Is there a change in primary outcome between registration and publication? | Outcomes | Reporting | |
Nùnẽz-Nùnẽz [56] 1 publication 5 questions |
Are there post publication comments, expressions of concern or retractions? | Search & selection | Governance |
| Does the data show integrity? | Data integrity | Governance | |
| Are there potential integrity concerns over contribution/authorship? | Data integrity | Governance | |
| Are there potential integrity concerns over conflict of interest? | Data integrity | Governance | |
| Are there potential integrity concerns over the funding source? | Data integrity | Governance | |
Sox and Rennie [57] 1 publication 1 question |
If an author has other article/s shown to be fraudulent, has someone close to the work (e.g., a coauthor) explained the specific reasons why he or she can vouch for its integrity? | Governance | |
Weibel et al. [58] 1 publication 25 questions |
Is there a retraction or expression of concern? | Studies retracted/expression of concern | Governance |
| Is the trial registration number reported? | Prospective trial registration | Governance | |
| Is the trial registered? | Prospective trial registration | Governance | |
| Is there consistency between the study dates reported in the publication and those reported in the registration documents? | Prospective trial registration | Governance | |
| Is the ethics committee approval reported? | Ethics approval | Governance | |
| Is the ethics committee named? | Ethics approval | Governance | |
| Is the ethics committee location given? | Ethics approval | Governance | |
| Is the ethics committee approval number reported? | Ethics approval | Governance | |
| Is written informed consent reported? | Ethics approval | Governance | |
| Is the ethics committee approval obtained by a nationally recognized ethics committee as defined in the country's clinical trial regulations? | Ethics approval | Governance | |
| Is there consistency between author affiliations and study countries? | Author group | Plausibility | |
| Is there author and study country consistency within different parts of the article? | Author group | Plausibility | |
| Are there a plausible number of authors for the trial? (e.g., single author for RCT is implausible) | Author group | Plausibility | |
| Is there sufficient reporting of the study design? | Methods | Reporting | |
| Is it clear that the trial is properly randomized? | Methods | Reporting | |
| Is the number of participants in group consistent with the reported method? | Methods | Plausibility | |
| Are baseline details reported in sufficient detail to assess whether randomization worked properly? | Methods | Reporting | |
| Is there a plausible number of patients with condition recruited within the timeframe? | Results | Plausibility | |
| Is there a realistic response rate? | Results | Plausibility | |
| Is there a realistic loss of follow-up? | Results | Plausibility | |
| Is it free from plagiarism? | Results | Plagiarism | |
| Is it free of excessive similarity/difference in participant characteristics between groups? | Results | Plausibility | |
| Is it free from discrepancies between data in figures, tables, or text? | Results | Reporting | |
| Is the study free from calculation errors? | Results | Statistics | |
| Are the results plausible (e.g., not a massive risk reduction)? | Results | Plausibility | |
Initially our aim was to have only two mutually exclusive domains (governance and plausibility). To avoid ambiguity, we added the third domain “statistics,” but even then some overlap was difficult to avoid, particularly between “statistics” and “plausibility.” Our guiding principle was to assign the question as “statistics” when some additional statistical analysis of the available data was needed. In the final iteration, for ease of use and further avoidance of ambiguity, we added two more distinct domains (plagiarism and reporting).
The table also shows the domain finally agreed on by us (governance with 92 questions, plausibility with 85, plagiarism with 13, reporting with 45 and statistics with 49 questions).
To avoid duplication and ambiguity, from the 284 original questions we then framed 77 summary questions and grouped them in 5 domains: governance (13 questions grouped in five subdomains), plausibility (17 questions grouped in five subdomains), plagiarism (four questions), reporting (29 questions grouped in six subdomains), and statistics (14 questions in four subdomains) (Table 2).
| GOVERNANCE |
| Article and/or author alerts, retractions, amendments, expressions of concern |
|
| Authorship integrity |
|
| Ethics |
|
| Prospective trial registration |
|
| Guidelines |
|
| PLAUSIBILITY |
| Using common sense, your medical knowledge, and given the timeframe and the locality, are the following plausible and realistic? |
| Workload |
|
| Participant characteristics |
|
| Randomization |
|
| Withdrawals/losses to follow up |
|
| Results |
|
| PLAGIARISM |
|
| REPORTING |
| Alerts |
|
| Authors |
|
| Study design |
|
| Sufficient reporting |
|
| Consistent reporting |
|
| Writing style |
|
| Communication with authors |
|
| STATISTICS |
| Correct and complete data |
|
| Consistent data |
|
| Statistical evidence of data integrity |
|
| Individual patient data (IPD) |
|
- Note: Questions in bold can be answered using “objective evidence” while others are to large extent a matter of judgment.
We judged 23 out of the 77 questions (30%) to be objective; that is, we agreed that they could be answered with a clear YES or NO answer backed with available written evidence (Table 2).
4 DISCUSSION
Our review of various attempts to assess the trustworthiness of published clinical trials revealed 77 questions grouped in five distinct domains (governance, plausibility, plagiarism, reporting, and statistics), shown in Table 2.
The questions in the “reporting” domain that include inadequate compliance with publication guidelines, poor command of language and sloppy editorial work are an explicit acknowledgment that that the authors may not have done something deliberately wrong. It may be difficult to distinguish between poor-quality research or outright fraudulent research or poor-quality reporting of good work. This is why, particularly for this domain, it is especially important to reach out to authors, as further communication may satisfy the reviewers that the data can be trusted. Paper mills are not mentioned in this domain definition or in Table 2 because their red flags (suspect journals, implausibility of the paper, plagiarism, use of AI) are not specific to paper mills.
Our view is that the answers to the majority of questions (about 70%) can be regarded as subjective, that is, not necessarily based on firm, easily verifiable, or publicly available evidence. This subjectivity is an important aspect of trustworthiness assessment that has to be acknowledged and dealt with appropriately. By appropriately we mean that every effort should be made to avoid biased assessments, such as ensuring independent assessment by more than one person, all of them free from conflict of interests. Equally important is full transparency and public disclosure of the reasoning why decisions on the lack of trustworthiness have been made.
Some of the questions can be answered relatively quickly and easily by someone with no specialist knowledge. Others will need clinical (content), statistical, or IT specialists. Many will need all of these working together. For instance, an isolated statistical analysis without clinical context could lead to a wrong answer; likewise, for some “plausibility” questions, statistical as well as clinical expertise may be needed for proper assessment. When individual patient data are available, another raft of specialist assessments will normally be required.
Most of the assessments in this paper don't directly address the relatively new problem of AI-generated fraudulent text, data, or images, which is threatening to overwhelm the entire peer review process [16, 17]. Some questions in the domains of reporting and plausibility can help here but much more work needs to be done on detecting this type of fraud. There are many publications focused on AI in the creation/detection of fraudulent studies [18, 19] but as this is such a fast-moving area of methodology we did not specifically search for such tools in our review. Rather, we urge that any trustworthiness assessor should check the most up-to-date literature and/or work with IT specialists to ensure that the latest methods are used. Using the principle of “it takes a thief to catch a thief,” the most useful asset in detecting AI-generated trustworthiness issues may soon be AI itself.
Please note that our summary (Table 2) is not a trustworthiness tool in itself, but a list of conceptual domains; an aide-memoire summing up the trustworthiness questions from Table 1. We acknowledge that some questions in Table 2 are quite broad, as an attempt to summarize all the items which have been proposed. As this field is moving quickly, for any future trustworthiness assessment work we urge interested parties to search for additional tools that will have appeared since this paper was published.
In our view, the trustworthiness assessment of clinical research will never be “one size fits all.” The strength of our work is that it provides a menu of domains and related questions that peer-reviewers, editors, systematic reviewers and developers of guidelines may wish to choose from, depending on the available time and resources. We argue that a fully transparent unbiased trustworthiness assessment that is limited in terms of domains and questions (not quality) is better than none. That said, we sincerely hope that this menu of domains and questions will encourage methodologists in validation work to identify the domains and questions that should be considered mandatory, those that are optional depending on the resources available, and those that could be discarded because of lack of discriminatory power.
AUTHOR CONTRIBUTIONS
Zarko Alfirevic designed the concept. Both authors worked on the data extraction and wrote the manuscript jointly.
ACKNOWLEDGMENTS
The authors have nothing to report.
APPENDIX A: The full list of 33 potential IPD checks given in the INSPECT-SR checklist (13). These were subsumed into the umbrella question “Do tests suggest that IPD has integrity”? in both Tables 1 and 2
- 1.
Inspect recruitment over calendar time (compare between groups)
- 2.
Identifying inliers using Mahalanobis distance
- 3.
Identifying inliers using singular value decomposition
- 4.
Statistical test to compare variances in baseline variables between groups using IPD (Levene, Brown-Forsythe)
- 5.
Make star plots for each group
- 6.
Check whether the randomization sequence consistent with the description in the paper
- 7.
Can the results in the paper be reproduced from the underlying data set?
- 8.
Plot differences between consecutive column values in order provided by author and by group (check for repetition, patterns, differences in patterns between groups)
- 9.
Compare distribution of leading digits in individual participant data between randomized arms
- 10.
Examine relationships between variables for biological plausibility (e.g., by plotting against each other)
- 11.
Are data internally consistent?
- 12.
Color code values in Excel spreadsheet to highlight outlying values, patterns and repetition
- 13.
Inspect time between participant visits
- 14.
Change global format to “general” in Excel—calculated values display long strings of numbers to right of decimal place, fabricated values may not
- 15.
Calculate autocorrelation between column values, overall and by group
- 16.
Test of runs of the same value (e.g., resampling, Wald-Wolfowitz)
- 17.
Compare kurtosis of baseline variables between groups
- 18.
Comparing multiple versions of a spreadsheet containing study data for consistency
- 19.
Statistical test to compare multivariate correlations between variables between treatment groups (several methods)
- 20.
Plot correlation coefficients for each pair of variables by group using greyscale/heatmap
- 21.
Consider whether distribution of variable follows simple but implausible model (such as Normal)
- 22.
Compare distribution of non-first digits in individual participant data between randomized arms
- 23.
Probablility of column sequences via simulation and resampling
- 24.
Plot column values in order provided by author and by group (check for repetition, patterns, differences in patterns between groups)
- 25.
Check visit dates (plausibility of visits on Sundays)
- 26.
Examining spreadsheet for formulae used to fabricate data
- 27.
Apply neighborhood clustering method of Wu and Carlsson
- 28.
Plot Chernoff faces for each group
- 29.
Compare leading digits in individual participant data to Benford's Law
- 30.
Compare non-first digits in individual participant data to expected
- 31.
Check repeated measures for interpolation and duplication
- 32.
Are only a small number of baseline characteristics collected in IPD?
- 33.
Checks of sequences in decimal places (after deleting integer)
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




