Unlocking Potential: Vegetable Oil Effects on Lactic Acid Extraction
ABSTRACT
The objective of this study was to recover lactic acid (LA) from aqueous solutions using a reactive extraction method with tri-n-octylamine (TOA) and vegetable-origin oils, including sunflower, corn, sesame, rice bran, olive, almond, palm, and canola oils as solvents. In physical extraction, sunflower oil gave the highest yield at 10.9 %, whereas reactive extraction followed a different trend. The maximum separation efficiency of 74.54 % was obtained when rice bran oil was used as the solvent at a TOA concentration of 3.61 mol kg−1. Distribution coefficient also reached its highest value (KD = 2.93) with rice bran oil as a solvent. It was found that the loading factors of the samples were inversely proportional to the increasing concentration of the reactant in the reactive extraction process. Principal component analysis shows that two components explain 93.5 % of the variance. Sesame oil and rice bran oil had the highest similarity (98.94 %) and were the closest group in the dendrogram.
1 Introduction
Carboxylic acids, essential to the food, chemical, and pharmaceutical industries, are a class of organic compounds widely used in the production of various industrial products, including animal feed, perfumes, textiles, dyes, plastics, pharmaceuticals, and detergents [1-3]. Additionally, carboxylic acids have found contemporary applications in fields such as biopolymer production, drug delivery, and tissue engineering [4, 5].
Lactic acid (LA), also known as 2-hydroxypropanoic acid, is a weak organic acid classified as an alpha-hydroxy acid due to its hydroxyl group adjacent to the carboxyl group. First isolated from milk by a Swedish chemist in 1870, LA was commercially produced for the first time in the United States in 1881. It can be produced both naturally (via fermentation) and synthetically (in vitro). Reports indicate that 82 % ± 1.5 % of LA is produced from organic waste through microbial fermentation [6]. LA is commonly derived from carbohydrates, such as corn, sugarcane, and wheat straw, through fermentation with bacteria or yeast [7]. Furthermore, LA is utilized in the production of propylene glycol, acrylic polymers, ethanol, and biodegradable and biocompatible polymeric materials [8]. To ensure optimal conditions for microorganism growth and production, the pH of the fermentation media must be controlled by adding calcium hydroxide, as the acid released during fermentation inhibits the production of carboxylic acids [9, 10]. However, the high demand for carboxylic acid production makes this recovery method both costly and environmentally harmful [10, 11]. Previous studies have explored various methods for recovering carboxylic acids from aqueous solutions, including membrane separation, electrodialysis, liquid–liquid extraction, ion exchange, adsorption, and reverse osmosis [12-14]. The separation methods mentioned above have several disadvantages, including high costs and energy consumption, the production of large amounts of wastewater and by-products, and low efficiency [13]. These methods require specialized chemicals and considerable energy to operate. For example, solvent extraction relies on expensive solvents, whereas membrane operations and reverse osmosis require significant energy to force water through membranes. Moreover, these processes often involve complex equipment and procedures, requiring skilled personnel and meticulous maintenance. All of these factors contribute to the overall high cost of implementing these separation methods [15]. In contrast, reactive extraction has gained attention for its various advantages in the recovery of organic acids from aqueous solutions, such as high purity and selectivity, low waste and by-product formation, and the ability to control the pH of the fermentation medium [13, 16-20]. Reactive extraction is a widely used separation process in industries like wastewater treatment and hydrometallurgy. This process involves a chemical reaction between a substance and a reactant, allowing the targeted substance to be extracted [21, 22]. The primary factor distinguishing reactive extraction from solvent extraction is the chemical reaction between the solute and the reactant in the organic phase.
Previous studies have shown that aliphatic amines and phosphorus compounds are important reactants in reactive extraction processes [23]. For instance, in the reactive extraction of pyruvic acid from a fermentation medium using vegetable oils, tri-n-octylamine (TOA) achieved the highest yield, with almond oil proving to be the most effective vegetable oil. Extraction yields improved with increasing amounts of extractant; however, high pH levels and pyruvic acid concentrations adversely affected the yield. The organic phases could be reused up to five times with only a 1–2 % decrease in yield [24]. Another study evaluated the use of various vegetable oils as solvents in the reactive extraction method to separate citric, malic, and glycolic acids from aqueous solutions, employing TOA as the extractant. The findings revealed that nearly 99 % of these acids could be separated from aqueous solutions using appropriate organic phase mixtures [25]. Soybean and rice bran oils, known for their non-toxicity and microorganism-friendly properties, were used for the reactive extraction of propionic acid from fermentation broth. Soybean oil demonstrated a higher distribution coefficient (0.73) and extraction yield (42.25 %) compared to rice bran oil, making these oils suitable for use in biorefinery industries due to their cost-effectiveness and environmentally friendly nature [26]. The process of separating acrylic acid from aqueous solutions using edible oils through reactive extraction was also investigated. High efficiency was observed, especially when substances, like TOA and TBP, were used; for example, extraction yields exceeding 90 % were achieved with safflower oil. Additionally, the use of modifiers such as octanol in the organic phase positively influenced separation efficiency [27]. An environmentally friendly reactive extraction method was employed to extract LA from an aqueous solution using green solvents. A system containing sunflower oil, TOA, and Aliquat336 achieved high efficiency in separating LA [28]. Another study examined the reactive extraction of LA from alkaline aqueous solutions, highlighting the increased efficiency obtained using carbonate Aliquat 336 in conjunction with polar-aprotic solvents such as MIBK. This method presents a promising approach for recovering LA from black liquor in pulp production [29]. The results obtained from the reactive liquid–liquid extraction method for the recovery of LA from fermentation broth showed that the most effective extraction was achieved using a solution containing 15 % TOA, 35 % TBP, 15 % Aliquat 336, and 35 % sunflower oil in the organic phase, resulting in extraction yields of up to 70 %. Finally, LA was successfully separated from the organic phase with a recovery rate of 90 % using a 0.5 M sodium carbonate solution [30].
Limited studies have been conducted on the recovery of LA from aqueous solutions using a reactive extraction method with solvents derived from vegetable sources [28, 31]. The most distinctive feature of our study is the use of eight different non-toxic vegetable oils. This study investigates the separation of LA from aqueous solutions through a reactive extraction method utilizing vegetable oil solvents. Specifically, eight different oils (sunflower, corn, sesame, rice bran, olive, almond, palm, and canola oils) were employed as solvents, whereas tri-n-octylamine (TOA) was used as the extractant. Although TOA is an effective reactant for recovering LA from aqueous solutions, it is inherently toxic. Additionally, conventional solvents are both toxic to microorganisms and more expensive compared to edible oils [32]. Therefore, vegetable oils were chosen as solvents for LA recovery due to their environmentally friendly properties and lower cost. This study aims to calculate the distribution coefficient (KD), loading factor (Z), and extraction yield (E %) resulting from the reactive extraction of LA using edible oils as solvents with TOA and to compare these results with those obtained through physical extraction.
2 Materials and Methods
2.1 Material
DL-LA was used as carboxylic acid in the reactive extraction method, whereas tri-n-octcylamine (TOA) was employed as the extractant, as shown in Tab. 1. Refined vegetable oils (sunflower, corn, sesame, rice bran, olive, almond, palm, and canola oils) were purchased from a local market and used as solvents in the reactive extraction process. Sodium hydroxide pellets and phenolphthalein indicator (pH) were obtained from Sigma-Aldrich. All chemicals were used as supplied, without any additional purification.
| Chemicals | CAS number | Molecular formula | Molecular structure | Molecular weight | Supplier | Purity (%) |
|---|---|---|---|---|---|---|
| Tri-n-octylamine | 1116-76-3 | [CH3(CH2)7]3N | 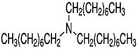 |
353.67 | Sigma-Aldrich | 98 |
| DL-lactic acid | 50-21-5 | CH3CH(OH)COOH | 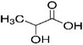 |
90.08 | Sigma-Aldrich | 90 |
| Sodium hydroxide | 1310-73-2 | NaOH | 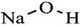 |
40.00 | Sigma-Aldrich | ≥98 |
2.2 Method
2.3 Chemometric Analysis
Multivariate data analysis was performed by applying spectral data to principal component analysis (PCA) and hierarchical cluster analysis (HCA). The main objective was to distinguish differences between the samples and the methodologies used. Data analysis was carried out using Minitab 18 software (Minitab Inc.). The PCA results were represented in a score plot and biplot, whereas the HCA employed Euclidean distance and single linkage for clustering.
3 Results and Discussion
In this study, the extraction efficiency, distribution coefficient (KD), and loading factors (Z) of LA from aqueous solutions were investigated using tri-n-octylamine (TOA) and environmentally friendly vegetable-origin solvents. According to numerous studies, the main factors affecting reactive extraction are the concentrations of the reactant and the acid [36, 37]. In this study, the initial LA concentration was set at 8 % (v/v) because the amount of LA produced in the fermentation medium is typically less than 10 % [38].
3.1 Physical Extraction
The strength of the hydrogen bonds formed between acid and water molecules surpasses that of the intermolecular hydrogen bonds formed exclusively among acid molecules. Therefore, carboxylic acids are generally present as monomers in aqueous solutions. Weaker hydrogen bonds between water and acid molecules facilitate greater access to acid molecules in solution [39]. Physical extraction studies of aqueous LA solutions were performed using various vegetable oils as solvents, including canola, sesame, almond, soybean, rice bran, olive, sunflower, and corn oils. The distribution coefficient (KD) and extraction efficiency (E) results of LA with different solvents are summarized in Tab. 2.
| Solvents | CLA(aq) (mol kg−1) | CLA(org) (mol kg−1) | KD | E % |
|---|---|---|---|---|
| Sunflower oil | 2.70 | 0.33 | 0.12 | 10.90 |
| Sesame oil | 2.89 | 0.14 | 0.05 | 4.47 |
| Rice bran oil | 2.89 | 0.13 | 0.05 | 4.45 |
| Olive oil | 2.97 | 0.06 | 0.02 | 1.96 |
| Almond oil | 2.89 | 0.14 | 0.05 | 4.56 |
| Palm oil | 2.96 | 0.06 | 0.02 | 2.07 |
| Canola oil | 2.95 | 0.08 | 0.03 | 2.50 |
| Corn oil | 2.89 | 0.14 | 0.05 | 4.52 |
Among the eight different solvents, sunflower oil exhibited the highest distribution coefficient (KD = 0.12), whereas olive and palm oils showed the lowest values (KD = 0.02) at an initial LA concentration of 3.03 mol kg−1. In terms of extraction yields, sunflower oil achieved the highest yield (10.9 %), whereas olive oil had the lowest yield (1.96 %). A higher distribution coefficient in solvents is observed when the interactions between solute and solvent molecules are stronger than the interactions between solute molecules themselves [23]. Sunflower oil demonstrated a slightly enhanced capacity for LA separation, attributed to its ability to form hydrogen bonds with LA molecules and mitigate the formation of LA dimers. Based on the findings from the physical extraction study, it can be inferred that sunflower oil, almond oil, corn oil, sesame oil, and rice bran oil are comparatively more effective solvents for separating LA from aqueous media than palm, canola, and olive oils. This distinction is likely due to the weaker interactions and lower solubility of palm, canola, and olive oils as diluents. For instance, olive oil is rich in oleic acid, which may not form as strong hydrogen bonds with LA as linoleic acid, leading to lower extraction efficiency. The results from the physical extraction indicate that these solvents, when used in isolation, do not provide sufficient extraction capabilities for effectively separating LA from aqueous media. Therefore, the inclusion of reaction enhancers, such as TOA, is essential to improve the recoverability of LA from aqueous solutions.
3.2 Reactive Extraction
The TOA concentration ranged from 5 % to 45 % (v/v) of the total volume in various solvents, whereas the initial LA concentration was kept constant at 3.03 mol kg−1. The findings related to the isolation of LA from aqueous solutions using TOA and vegetable-derived solvents have been thoroughly examined, covering essential extraction parameters, and are presented in Tab. 3.
| Solvents | [TOA]org (mol kg−1) | CLA(aq) (mol kg−1) | CLA(org) (mol kg−1) | KD | Avg. KD | Z | E % | Avg. E % | Ratio (KD1/KD0) |
|---|---|---|---|---|---|---|---|---|---|
| Sunflower oil | 0.00 | 2.70 | 0.33 | 0.12 | 1.50 | 10.90 | 52.46 | 1.00 | |
| 0.40 | 2.48 | 0.54 | 0.22 | 1.36 | 18.00 | 1.79 | |||
| 0.81 | 1.88 | 1.15 | 0.61 | 1.41 | 37.98 | 5.01 | |||
| 1.21 | 1.48 | 1.55 | 1.05 | 1.28 | 51.13 | 8.55 | |||
| 1.60 | 1.23 | 1.79 | 1.45 | 1.12 | 59.25 | 11.89 | |||
| 1.98 | 1.04 | 1.99 | 1.92 | 1.00 | 65.70 | 15.66 | |||
| 2.37 | 0.98 | 2.04 | 2.08 | 0.86 | 67.58 | 17.04 | |||
| 2.90 | 0.93 | 2.10 | 2.27 | 0.72 | 69.38 | 18.53 | |||
| 3.16 | 0.88 | 2.14 | 2.43 | 0.68 | 70.87 | 19.89 | |||
| 3.62 | 0.79 | 2.23 | 2.82 | 0.62 | 73.82 | 23.06 | |||
| Sesame oil | 0.00 | 2.89 | 0.14 | 0.05 | 1.50 | 4.47 | 51.07 | 1.00 | |
| 0.42 | 2.77 | 0.26 | 0.09 | 0.62 | 8.57 | 2.00 | |||
| 0.78 | 1.83 | 1.20 | 0.65 | 1.53 | 39.54 | 13.97 | |||
| 1.18 | 1.49 | 1.53 | 1.03 | 1.31 | 50.69 | 21.96 | |||
| 1.60 | 1.25 | 1.77 | 1.42 | 1.11 | 58.59 | 30.24 | |||
| 1.99 | 1.11 | 1.92 | 1.73 | 0.97 | 63.43 | 37.06 | |||
| 2.39 | 0.97 | 2.05 | 2.11 | 0.86 | 67.84 | 45.08 | |||
| 2.77 | 0.87 | 2.16 | 2.48 | 0.78 | 71.30 | 53.08 | |||
| 3.20 | 0.85 | 2.18 | 2.58 | 0.68 | 72.07 | 55.13 | |||
| 3.54 | 0.78 | 2.25 | 2.88 | 0.63 | 74.25 | 61.63 | |||
| Rice bran oil | 0.00 | 2.89 | 0.13 | 0.05 | 1.69 | 4.45 | 55.08 | 1.00 | |
| 0.53 | 2.17 | 0.86 | 0.39 | 1.63 | 28.32 | 8.48 | |||
| 0.81 | 1.78 | 1.25 | 0.70 | 1.54 | 41.22 | 15.06 | |||
| 1.21 | 1.37 | 1.66 | 1.21 | 1.37 | 54.71 | 25.94 | |||
| 1.64 | 1.11 | 1.92 | 1.74 | 1.17 | 63.44 | 37.27 | |||
| 2.00 | 1.00 | 2.03 | 2.03 | 1.01 | 67.01 | 43.62 | |||
| 2.42 | 0.90 | 2.13 | 2.37 | 0.88 | 70.35 | 50.95 | |||
| 2.79 | 0.83 | 2.19 | 2.63 | 0.79 | 72.49 | 56.58 | |||
| 3.18 | 0.78 | 2.25 | 2.89 | 0.71 | 74.27 | 61.99 | |||
| 3.61 | 0.77 | 2.26 | 2.93 | 0.63 | 74.54 | 62.87 | |||
| Olive oil | 0.00 | 2.97 | 0.06 | 0.02 | 1.55 | 1.96 | 51.61 | 1.00 | |
| 0.41 | 2.60 | 0.43 | 0.17 | 1.06 | 14.21 | 8.29 | |||
| 0.80 | 1.89 | 1.13 | 0.60 | 1.42 | 37.45 | 29.98 | |||
| 1.22 | 1.51 | 1.51 | 1.00 | 1.24 | 49.96 | 49.99 | |||
| 1.60 | 1.23 | 1.79 | 1.45 | 1.12 | 59.23 | 72.73 | |||
| 2.01 | 1.09 | 1.94 | 1.78 | 0.97 | 64.09 | 89.34 | |||
| 2.48 | 0.93 | 2.09 | 2.25 | 0.85 | 69.21 | 112.54 | |||
| 2.85 | 0.82 | 2.21 | 2.69 | 0.78 | 72.93 | 134.86 | |||
| 3.19 | 0.81 | 2.21 | 2.71 | 0.69 | 73.07 | 135.86 | |||
| 3.55 | 0.79 | 2.24 | 2.85 | 0.63 | 74.02 | 142.63 | |||
| Almond oil | 0.00 | 2.89 | 0.14 | 0.05 | 0.98 | 4.56 | 42.40 | 1.00 | |
| 0.38 | 2.82 | 0.21 | 0.07 | 0.55 | 6.90 | 1.55 | |||
| 0.75 | 2.24 | 0.79 | 0.35 | 1.04 | 25.94 | 7.33 | |||
| 1.13 | 1.98 | 1.05 | 0.53 | 0.93 | 34.74 | 11.14 | |||
| 1.58 | 1.56 | 1.47 | 0.94 | 0.92 | 48.42 | 19.64 | |||
| 1.96 | 1.39 | 1.64 | 1.18 | 0.84 | 54.23 | 24.79 | |||
| 2.37 | 1.28 | 1.74 | 1.36 | 0.74 | 57.62 | 28.46 | |||
| 2.79 | 1.15 | 1.88 | 1.63 | 0.67 | 62.03 | 34.19 | |||
| 3.17 | 1.12 | 1.90 | 1.70 | 0.60 | 62.93 | 35.52 | |||
| 3.57 | 1.01 | 2.02 | 2.00 | 0.56 | 66.62 | 41.77 | |||
| Palm oil | 0.00 | 2.96 | 0.06 | 0.02 | 0.76 | 2.07 | 36.96 | 1.00 | |
| 0.40 | 2.93 | 0.09 | 0.03 | 0.23 | 3.03 | 1.48 | |||
| 0.82 | 2.50 | 0.53 | 0.21 | 0.65 | 17.40 | 9.96 | |||
| 1.23 | 2.06 | 0.96 | 0.47 | 0.78 | 31.84 | 22.09 | |||
| 1.61 | 1.76 | 1.26 | 0.71 | 0.78 | 41.69 | 33.81 | |||
| 1.97 | 1.61 | 1.41 | 0.88 | 0.72 | 46.75 | 41.51 | |||
| 2.40 | 1.45 | 1.57 | 1.08 | 0.66 | 51.99 | 51.21 | |||
| 2.79 | 1.32 | 1.71 | 1.30 | 0.61 | 56.50 | 61.43 | |||
| 3.21 | 1.26 | 1.77 | 1.40 | 0.55 | 58.35 | 66.25 | |||
| 3.57 | 1.21 | 1.82 | 1.50 | 0.51 | 60.01 | 70.97 | |||
| Canola oil | 0.00 | 2.95 | 0.08 | 0.03 | 1.01 | 2.50 | 42.32 | 1.00 | |
| 0.37 | 2.91 | 0.12 | 0.04 | 0.32 | 3.92 | 1.59 | |||
| 0.80 | 2.32 | 0.71 | 0.31 | 0.89 | 23.43 | 11.94 | |||
| 1.20 | 1.93 | 1.10 | 0.57 | 0.91 | 36.22 | 22.15 | |||
| 1.56 | 1.58 | 1.45 | 0.92 | 0.93 | 47.88 | 35.84 | |||
| 1.94 | 1.35 | 1.67 | 1.24 | 0.86 | 55.30 | 48.27 | |||
| 2.37 | 1.23 | 1.80 | 1.47 | 0.76 | 59.50 | 57.31 | |||
| 2.79 | 1.13 | 1.89 | 1.67 | 0.68 | 62.61 | 65.33 | |||
| 3.16 | 1.06 | 1.97 | 1.85 | 0.62 | 64.95 | 72.29 | |||
| 3.51 | 1.00 | 2.02 | 2.02 | 0.58 | 66.88 | 78.78 | |||
| Corn oil | 0.00 | 2.89 | 0.14 | 0.05 | 0.99 | 4.52 | 42.38 | 1.00 | |
| 0.43 | 2.80 | 0.23 | 0.08 | 0.52 | 7.45 | 1.70 | |||
| 0.77 | 2.35 | 0.68 | 0.29 | 0.88 | 22.44 | 6.12 | |||
| 1.18 | 2.02 | 1.01 | 0.50 | 0.86 | 33.34 | 10.57 | |||
| 1.57 | 1.55 | 1.48 | 0.96 | 0.94 | 48.93 | 20.26 | |||
| 2.02 | 1.33 | 1.69 | 1.27 | 0.84 | 55.98 | 26.88 | |||
| 2.36 | 1.21 | 1.81 | 1.50 | 0.77 | 59.92 | 31.61 | |||
| 2.78 | 1.16 | 1.87 | 1.61 | 0.67 | 61.67 | 34.02 | |||
| 3.14 | 1.10 | 1.93 | 1.76 | 0.61 | 63.75 | 37.19 | |||
| 3.56 | 1.03 | 1.99 | 1.93 | 0.56 | 65.81 | 40.70 |
[TOA]org is the tri-n-octylamine concentration in the organic phase. CLA(aq) and CLA(org) denote the LA concentration in the aqueous and organic phases, respectively. KD represents the distribution coefficient, whereas Avg. KD denotes the average distribution coefficient. Z denotes the loading factor in reactive extraction, and E % represents the efficiency of extraction. E % is the average efficiency of extraction. LA's KD1/KD0 ratio indicates the tendency of the substance to remain in solution under specific conditions compared with standard reference conditions.
The extraction efficiency and distribution of the acid are significantly influenced by the quantity of extractant present in the organic phase. TOA was combined with different diluents at various concentrations to identify the most effective combination of extractant and diluent. The maximum KD value of 2.93 was achieved with 3.61 mol kg−1 TOA dissolved in rice bran oil among all TOA-diluent combinations. Within the scope of the study, KD values were found to range between 0.931 and 12.333 during the reactive extraction process for propionic acid, where tri-n-octylamine was used in conjunction with 1-decanol as the extraction solvent [37]. The observed variation in KD values compared to our study can be attributed to the specific choice of solvent and the inherent chemical properties of the extracted substance. Figs. 1 and 2 depict the changes in the distribution coefficients and extraction efficiency as the concentration of the extractant increases. The efficiencies of LA extracted by TOA ranged from 3.03 % to 74.54 %. The highest separation efficiency, 74.54 %, was achieved using 3.61 mol kg−1 TOA with rice bran oil. Following rice bran oil, the sequence of solvents, in terms of decreasing separation efficiency, was sesame oil, olive oil, sunflower oil, canola oil, almond oil, corn oil, and palm oil. Rice bran oil, sunflower oil, and sesame oil have high extraction efficiencies and distribution coefficients, which can be explained by the strong interactions between LA and TOA due to their high unsaturated fatty acid content and appropriate polarity. On the other hand, canola oil, corn oil, and palm oil showed lower extraction efficiency and dispersion coefficients, suggesting that the chemical properties of these oils are not compatible with LA and have lower dissolution capacities. To summarize, the varying efficiencies observed with different vegetable oils can be attributed to a combination of their intrinsic chemical characteristics, compatibility with the extractant, intermolecular interactions, and the fine-tuning of operational parameters throughout the extraction process. In a similar study, the highest yield of 66 % was recorded for the reactive extraction of LA using TOA and safflower oil, which was achieved at the highest concentrations of TOA and LA. However, under the same conditions, a yield of 76 % was obtained when 1-octanol was used as the solvent [40]. Another study investigated a thymol–menthol-based deep eutectic solvent (tmDES), an environmentally friendly alternative to 1-octanol commonly used for the extraction of LA, along with pretreated sweet sorghum silage press juice (pSPJ). In this study, TOA was employed as a reactive extractant. The experiments demonstrated that using tmDES instead of 1-octanol at 50 °C, increased the maximum extraction efficiency for LA from 35.0 % ± 0.8 % to 48.7 % ± 0.7 % when pSPJ was used as the feed phase [41]. According to the findings, the environmentally friendly green solvents provided higher efficiency. Additionally, another study conducted a detailed examination of the extraction of LA from aqueous solution using an emulsion liquid membrane (ELM). Under optimized conditions and within a suitable range of specific operational parameters, over 95 % of LA was efficiently extracted within just 20 min. These results highlight the importance of optimizing both the components and operational parameters for formulating an effective ELM and achieving efficient LA extraction [42]. We can say that edible oils will be an important alternative solvent between water and phase-forming substances in terms of being cheap and biocompatible, as well as being low cost [43-46]. To evaluate the impact of vegetable-origin solvents on LA recovery from aqueous solutions via the reactive extraction method, the distribution coefficients and extraction yields were carefully analyzed. The maximum KD values obtained from the LA/TOA extraction system were ranked as follows: rice bran oil (KD = 2.93) > sesame oil (KD = 2.88) > olive oil (KD = 2.85) > sunflower oil (KD = 2.82) > canola oil (KD = 2.02) > almond oil (KD = 2.00) > corn oil (KD = 1.93) > palm oil (KD = 1.50). The order of extraction yields was found to be similar to that of the distribution coefficients.
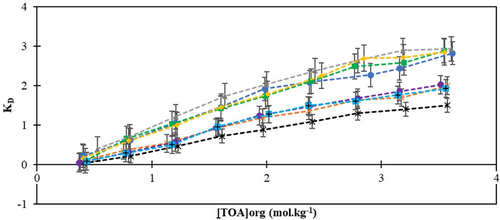
 , sunflower oil;
, sunflower oil;  , sesame oil;
, sesame oil;  , rice bran oil;
, rice bran oil;  , olive oil;
, olive oil;  , almond oil;
, almond oil;  , palm oil;
, palm oil;  , canola oil;
, canola oil;  , corn oil.
, corn oil.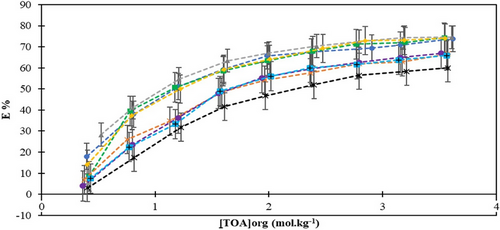
 , sunflower oil;
, sunflower oil;  , sesame oil;
, sesame oil;  , rice bran oil;
, rice bran oil;  , olive oil;
, olive oil;  , almond oil;
, almond oil;  , palm oil;
, palm oil;  , canola oil;
, canola oil;  , corn oil.
, corn oil.The loading factors (Z) obtained from aqueous solutions of LA using the reactive extraction method with vegetable-origin solvents are presented in Tab. 3 and Fig. 3. After performing extraction calculations, a loading factor of less than 0.5 indicates that each acid molecule can be complexed with only one extractant molecule, potentially resulting in a 1:1 acid-amine complex formation [47, 48]. According to the extraction results, the loading factor was greater than 0.5 in almost all cases. As reported by Demir et al., if the loading factor (Z) exceeds 0.5, overloading in the amine can be anticipated [23]. Therefore, a higher rate of complexation is likely when TOA is used as the reactant in reactive extractions. The literature identifies amines and organophosphorus compounds as the primary extractants employed for the separation of carboxylic acids from aqueous solutions [23]. Another study noted that as the concentration of TBP in [HMIM][PF6] and the initial concentration of propionic acid increased, the loading factor values ranged from 0.19 to 0.69. These findings suggest the presence of multiple phosphates in each complex [49]. Additionally, the observed relationship, where an increase in phase ratios leads to a decrease in the loading factor, reveals similar trends in the study. Shakya et al., found that an increase in the percentage of TBP during the reactive extraction of acetic acid resulted in a decrease in the loading factor, reaching its maximum at the highest acid concentrations [50]. Similarly, studies by Marti [51] and Uslu [52] yielded comparable Z values using natural diluents. The results indicate that increasing the reactant concentration in the reactive extraction process tends to decrease the loading factors. In summary, the extraction system presented in this study shows considerable potential for practical application and further expansion, particularly due to its eco-friendly nature, the diverse range of solvents used, the adoption of reactive extraction techniques, and its suitability for industrial purposes. However, additional research and development are needed to optimize the system for mass production and effectively address scalability challenges.
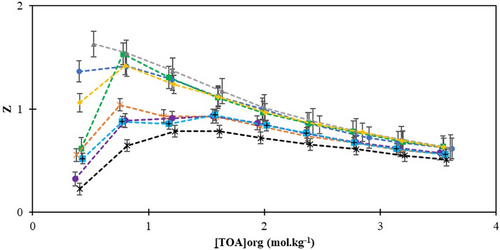
 , sunflower oil;
, sunflower oil;  , sesame oil;
, sesame oil;  , rice bran oil;
, rice bran oil;  , olive oil;
, olive oil;  , almond oil;
, almond oil;  , palm oil;
, palm oil;  , canola oil;
, canola oil;  , corn oil.
, corn oil.3.3 Chemometric Results
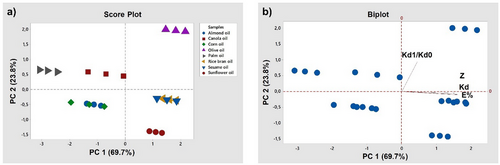
The categorization (extraction yield, distribution coefficient, loading factor, and ratio) of the reactive extraction method of LA using eight different environmentally friendly solvents was performed. According to the PCA score plot, the two principal components represented 93.5 % of the total variance (Fig. 4). PC1 explained 69.7 % of the total variance, whereas PC2 explained 23.8 %. Especially E % (0.582), Z (0.542), and KD (0.584) variables have high positive contribution on PC1. PC2 explains 23.8 % of the variance and has a particularly strong relationship with the KD1/KD0 variable (98.6 %). These components showed the main criteria by which the samples differed. Rice bran oil and sesame oil are located at the positive end of PC1, indicating that these oils are rich in the components associated with this axis. Corn oil is at the negative end of PC1, indicating that it has different constituent profiles than oils at the other end of this axis. Palm oil and canola oil are at the positive end of PC2. These oils may exhibit properties with higher values relative to this component. Almond oil is positioned at the negative end of PC2, indicating that it has lower values in the properties associated with this axis (Tabs. 4, 5).
| Variable | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| E % | 0.582 | −0.118 | 0.396 | 0.700 |
| Z | 0.542 | −0.061 | −0.838 | 0.013 |
| KD | 0.584 | −0.097 | 0.374 | −0.713 |
| KD1/KD0 | 0.161 | 0.986 | 0.033 | 0.015 |
| Eigenvalue | 2.7878 | 0.9536 | 0.2510 | 0.0076 |
|---|---|---|---|---|
| Proportion | 0.697 | 0.238 | 0.063 | 0.002 |
| Cumulative | 0.697 | 0.935 | 0.998 | 1.000 |
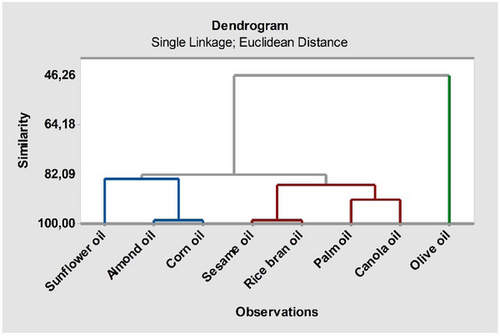
The dendrogram in Fig. 5 forms a clustering hierarchy based on distance between samples. A dendrogram is a tree structure that shows how data are grouped according to their similarities. The samples in the lower branches of the dendrogram are those that are most similar to each other. These samples are grouped in the same cluster and exhibit similar characteristics according to the reagent extraction data. The branches at higher levels show samples with lower similarities but still with common characteristics. This implies that the samples are grouped into broad clusters on a common basis. Fig. 5 shows that sesame oil and rice bran oil showed particularly high similarity (98.94 %), followed by almond oil and corn oil (98.88 %).
4 Conclusions
This study investigated the extraction efficiency, distribution coefficient (KD), and loading factors (Z) of LA from aqueous solutions using tri-n-octylamine (TOA) and environmentally friendly vegetable-origin solvents, including canola, sesame, almond, soybean, rice bran, olive, sunflower, and corn oils. In all cases, the loading factor values were above 0.5, indicating significant overloading of the extracts and suggesting a higher potential for complexation in extractions involving tri-n-octylamine. In the recovery of LA from aqueous solutions by the reactive extraction method, the maximum separation efficiency of 74.54 % was achieved when rice bran oil was used as the solvent at a TOA concentration of 3.61 mol kg−1. Regarding the distribution coefficient, the highest value (KD = 2.93) was also obtained with rice bran oil as the solvent. The findings suggest that the use of an extractant like TOA significantly enhances both the distribution coefficient and extraction efficiency. This improvement is attributed to the more effective transfer of acid to the organic phase, facilitated by the presence of the extractant in the organic phase. Studies of this nature can contribute to the development of environmentally friendly and efficient processes for industrial applications. According to the PCA results, PC1 and PC2 explained 93.5 % of the total variance, with sesame oil and rice bran oil showing high similarity (98.94 %). Furthermore, the dendrogram analysis indicated that the samples were clustered based on their similarities, particularly highlighting that sesame oil and rice bran oil were the closest to each other.
Author Contributions
Mustafa Kanbur: Investigation; data curation. Mehmet Yetisen: Investigation; data curation; writing—review and editing. Hasan Uslu: Supervision; project administration; conceptualization; methodology; investigation; data curation; writing—review and editing.
Conflict of Interest
The authors declare that they have no conflicts of interest or personal relationships that could have influenced the work reported in this paper.




