Local Mass Transfer Phenomena and Chemical Selectivity of Gas-Liquid Reactions in Capillaries
W. Krieger*, J. Lamsfuß, W. Zhang, N. Kockmann, Chem. Eng. Technol. 2017, 40 (11), 2134–2143. DOI: https://doi.org/10.1002/ceat.201700420
The article reported on a color reaction for determining selectivity in gas-liquid reactions. The stoichiometric values were taken from literature [8] and integrated into the calculations and discussion. Current own stoichiometry and mass balance measurements of the oxidation of leuco-indigo carmine lead to corrected values of a single oxygen molecule only needed for each oxidizing step, shown below. Particularly, the mass transfer coefficients must be corrected towards higher values. The authors apologize for the error and want to correct the publication as follows:
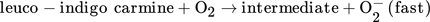 (3)
(3)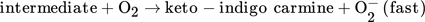 (4)
(4)Fig. 1 should be as shown below. Leuco-indigo carmine occurs in its deprotonated state due to the alkaline solution.
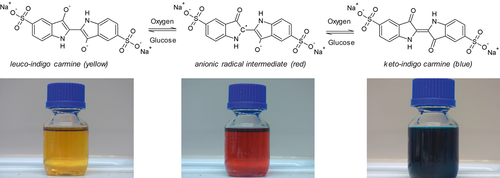
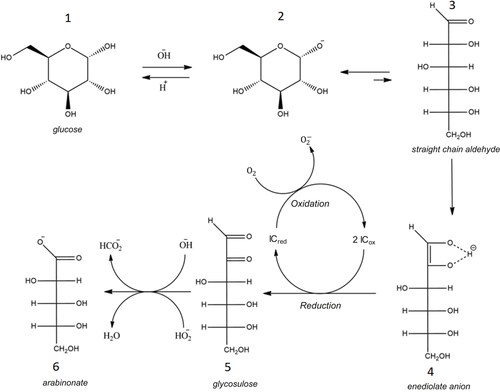
Fig. 2 should be as shown below according to the corrected stoichiometry. The proposed mechanism is supported by [17].
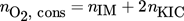 (11)
(11)Figs. 10 and 11 should be as shown below due to the corrected stoichiometry.
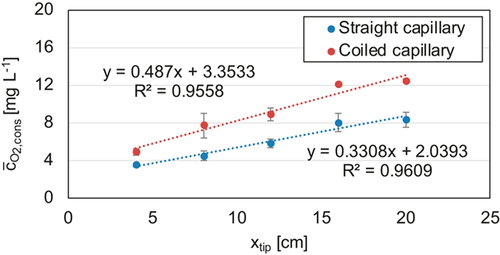
 = 3 mL min−1 and gas-to-liquid flow ratio of 1.0 in a straight and coiled capillary with an inner diameter of 1.6 mm.
= 3 mL min−1 and gas-to-liquid flow ratio of 1.0 in a straight and coiled capillary with an inner diameter of 1.6 mm.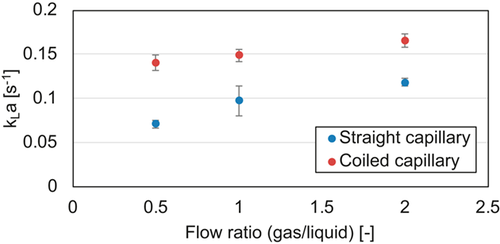
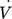 = 3 mL min−1 in a straight and curved capillary with an inner diameter of 1.6 mm.
= 3 mL min−1 in a straight and curved capillary with an inner diameter of 1.6 mm.On page 2141, right column, line 7 should read “The attained kLa values range from 7.08 to 16.52 × 10−2 s−1 and are comparable to data from the literature for microchannels and capillaries [22, 23].”
On page 2142, left column, lines 22–24 should read “Mass transfer investigations led to kLa values for straight and coiled capillaries in the order of 5–20 × 10−2 s−1, which are in reasonable agreement with data from literature.”




