A superficial siderosis-related transient focal neurological episode in a patient with Marfan syndrome
Edited by Yi Cu
Graphical Abstract
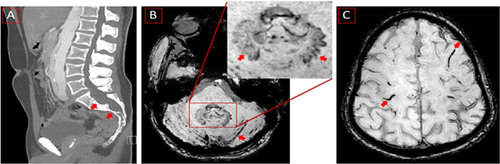
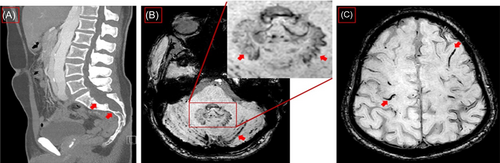
Sagittal computed tomography of lumbal spine showing dural ectasia and Tarlow Cyst (red arrows) (A). Magnetic resonance imaging (MRI) axial slice, susceptibility-weighted imaging (SWI) sequences showing siderosis in cerebellar sulci (red arrows) and in the inset uncharacteristic iron deposition in dental nuclei, more pronounced on the left side (red arrows) (B, inset). MRI, SWI, axial slice, showing numerous foci of superficial siderosis (C).
Marfan syndrome is an autosomal dominant disorder of connective tissue caused by mutations in the gene encoding a matrix component of microfibrils; fibrillin-1 (FBN1),1 with an incidence of about 1–3 per 10,000 individuals.2, 3 Cardinal manifestations include proximal aortic aneurysm, dislocation of the ocular lens, and long-bone overgrowth.2 Dural ectasia, which is defined as a ballooning or significant widening of the dural sac or neural root sleeves, is also a common finding in these patients, which is reported to present in more than 60% of cases and is considered a major criterion for the clinical diagnosis of this syndrome.3
Superficial siderosis (SS), on the other hand, is a rare condition of hemosiderin accumulation in the central nervous system resulting from prolonged low-grade bleeding into the subarachnoid space.4 Cerebral irritation due to cortical SS can lead to transient focal neurological deficits.5 Arteriovenous malformations (AVM), head or spinal trauma, prior neurosurgery, and tumors of the central nervous system are usual culprits. Furthermore, cortical (and to a lesser extent infratentorial) SS is a known complication of cerebral amyloid angiopathy (AA) in persons over 55 years of age. In a younger population, and without AVM, tumor, or familial AA traits, despite extensive imaging, the source of bleeding is often not evident during the evaluation.6 Rarely, in the cases presenting with a chronic cerebrospinal fluid leak, the cause of SS could be underlying dural defects in such patients.7
A 38-year-old man was admitted due to temporary numbness that suddenly appeared the day before along the entire medial aspect of the left upper extremity and lasted for about 20 min (NIHSS 0, mRS 0). Brain MRI showed no evidence of recent ischemia or intracranial aneurysms but cortical hemosiderin deposition characteristic of SS (Figure 1). Extensive dural ectasia was also detected in thoracolumbar computed tomography (CT) (Figure 1). Neurosonography showed slight arteriosclerotic wall changes without relevant plaque formation. No relevant abnormalities were detected in echocardiography. Long-term ECG had no evidence of atrial fibrillation. There were also no deposits on the mechanical aortic valves in transesophageal echocardiography. The CT angiography showed no main trunk occlusion and an inconspicuous display of the internal carotids. The descending thoracic aorta measured almost 4 cm, showing an existing dissection membrane with a small true lumen at 32 cm, two small perforations approximately 12 mm apart, the larger one with a diameter of 4 mm with shunt depending on the cardiac cycle in both directions. The aorta also showed a dissection after the aortic prosthesis at the beginning of the aortic arch and reaching into the pelvic vessels. The dissection continued into the brachiocephalic trunk, the right common carotid artery, and the left common carotid artery. No indication of a shunt or patent foramen ovale was found. Findings were considered clinically nonsignificant by the cardiac surgeon who performed the operation.
In the history, the surgical background was positive for Stanford A aortic dissection with a mechanical Bentall procedure (composite graft replacement of the aortic valve, aortic root, and ascending aorta) about one and half years before the presentation because of increasing aneurysmal expansion in the aortic arch. A mechanical aortic valve (27 mm St. Jude conduit) was implanted, as well as partial replacement of the aortic arch using hemiarch (28 mm Gelweave prosthesis). He was anticoagulated with Marcoumar. At the time of admission, the international normalized ratio was 2.2, and he was receiving antihypertensive medication (amlodipin/valsartan 5 mg/80 mg twice daily and bisoprolol 5 mg 1/2 daily) in addition to atorvastatin 20 mg daily.
We presented an intriguing association of transient focal neurological deficit with cortical SS in a 38-year-old patient with the diagnosis of Marfan syndrome for whom aortic dissection repair had been done with implantation of the mechanical aortic valve and partial replacement of the aortic arch.
Bower et al. in 2018 reported a similar case in a 67-year-old woman with a history of Marfan syndrome who presented with a fall.8 Dural ectasia and a large Tarlov cyst extruding through the sacral foramina were detected in the thoracic and lumbar spine, and brain MRI was compatible with the diagnosis of SS, in addition to her history and physical examination. They proposed Marfan syndrome as a probable cause of SS through the process of dural abnormality development and gradual deposition of hemosiderin due to chronic bleeding of these abnormalities into the subarachnoid space. There are several distinct differences between the case described by Bower et al. and our case: (1) the advanced age of Bower's patient, raising the question of whether Cerebral amyloid angiopathy (CAA) was a probable cause of siderosis; (2) the clinical presentation dominated by sensorineural deafness; (3) the imaging showed no cerebellar sulcal involvement as in our patient, and finally (4) our patient had a major aorto-valvular surgical repair and was under chronic anticoagulation.
SS of the central nervous system, which is the result of chronic recurrent hemorrhages (e.g., AVMs, tumors, or trauma), may lead to the accumulation of cytotoxic hemosiderin in the subarachnoid space.9 A history of prior intradural surgery or trauma also is usually common.10 Unlike in our case, the patients with infratentorial SS usually present with slowly progressive and irreversible cerebellar ataxia, sensorineural hearing loss, and myelopathy.6
The reported prevalence of dural pathology in patients diagnosed with SS ranges from 47% to 56%.4, 11 On the other hand, dural ectasia, which is a common sequel of connective tissue dysfunction, is reported to be present in 63%–92% of adult patients with Marfan syndrome.11
An important study of 30 cases of SS showed dural defects in 17 patients, and evidence of active bleeding such as xanthochromia in the cerebrospinal fluid and elevated RBC counts were found in 14 and 6 patients, respectively. The authors, therefore, proposed “duropathies” as a potential cause of chronic bleeding in SS.11 In another series of 65 cases of SS from the United Kingdom, dural abnormalities were detected in 40 patients.12 Dural ectasia without an obvious dural defect could also be present in patients with Marfan syndrome, neurofibromatosis, and ankylosing spondylitis with SS.12 In some observations with perioperative endoscopy, fragile and bleeding bridging veins have been noted to run between the dural layers.13 Exudation of blood from these damaged and engorged epidural or intradural vessels has been speculated to be a possible source of chronic bleeding that results in SS.13
We hypothesize that the co-occurrence of dural ectasia in the background of Marfan syndrome in our patient could be the potential cause of chronic microhemorrhages leading to the development of cortical SS. In addition, microvascular traumas from mechanical aortic valve and aortic arch replacement in a patient who is under antihypertension and anticoagulation therapy would probably make him susceptible to developing fragile and bleeding dural tears as the possible source of chronic bleeding that gradually developed SS. However, the existence of dentate hemosiderin deposition remains unclear (Figure 1). Hemosiderin deposition is usually a hallmark of several neurodegenerative conditions that involves deposition in the basal ganglia and substantia nigra.
Treatment of SS involves identification and surgical correction of the bleeding source, in addition to Deferiprone, which is a lipid-soluble iron chelator that can penetrate the blood–brain barrier and is reported to be effective in improving clinical symptoms.6 However, the currently available data might still be insufficient to recommend the routine use of Deferiprone in patients with SS.14
Transient focal neurological episodes (TFNEs) are caused by a hemisoderin deposition along the cortical surface that exerts an irritating effect on neurons.5 Important differential diagnoses are epileptic seizures and transitory ischemic attacks (TIA). Although TIA was suspected in our patient due to mechanical aortic valve replacement, the young age of our patient, absence of additional vascular risk factors, absence of acute dissection, and sufficient anticoagulation precluded the diagnosis of TIA. TFNEs are known to arise from cortical SS, however, mostly in the setting of known CAA—the diagnosis which was deemed highly unlikely in our patient due to his young age and absence of typical cortical microbleeds. On the other side, our patient had a singular episode of neurological deficit. Singularity does not fit into the clinical hallmark of TFNEs, which have spreading onset and stereotyped attacks. A focal sensory epileptic seizure could be another possibility in our patient. However, EEG showed no abnormality in our patient.
We reported disseminated SS and spinal dural ectasia in a patient presenting with transient focal neurological deficit carrying Marfan syndrome for whom aortic dissection repair with implantation of the mechanical aortic valve and partial replacement of the aortic arch had been done. Several underlying vascular abnormalities existing in this patient in the context of Marfan syndrome seem to be the most reasonable cause of this presentation. We recommend patients with connective tissue disorders such as Marfan syndrome should be monitored more frequently regarding the development of SS as these could be the cause of transitory neurological deficits.
AUTHOR CONTRIBUTIONS
Slaven Pikija conceptualized the study. Mahdi Safdarian, Slaven Pikija, Eugen Trinka, and Andreea Toma drafted the manuscript. Slaven Pikija, Mahdi Safdarian, and Eugen Trinka had access and verified the data. Slaven Pikija, Mahdi Safdarian, Eugen Trinka, Pfaff Johannes, and Andreea Toma contributed to reviewing and finalizing the manuscript. Mahdi Safdarian had the final responsibility to submit for publication. All other coauthors provided critical revisions of the manuscript and its supplement and approved the final version of the manuscript.
ACKNOWLEDGMENT
Not applicable.
CONFLICT OF INTEREST STATEMENT
Eugen Trinka reports personal fees from EVER Pharma, Marinus, Arvelle, Argenix, Medtronic, Bial-Portela & Ca, Newbridge, GL Pharma, GlaxoSmithKline, Boehringer Ingelheim, LivaNova, Eisai, UCB, Biogen, Genzyme Sanofi, and Actavis. His institution received grants from Biogen, UCB Pharma, Eisai, Red Bull, Merck, Bayer, the European Union, FWF Osterreichischer Fond zur Wissenschaftsforderung, Bundesministerium für Wissenschaft und Forschung, and Jubiläumsfond der Österreichischen Nationalbank outside the submitted work. Other authors have no conflicts of interest to declare that are relevant to the content of this article.
ETHICS STATEMENT
The internal Ethics committee of the Department of Neurology, Neurocritical Care and Neurorehabilitation, Christian Doppler University Hospital, Centre of Cognitive Neuroscience, Paracelsus Medical University approved this paper. Informed consent was taken from the patient for anonymously using his data for publication.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.