Determinants of survival outcomes among esophageal cancer patients at a national referral hospital in Kenya
Edited by Yi Cui
Abstract
Introduction
The overall 5-year survival rate for esophageal cancer patients in low- and middle-income countries was reported to be low, despite the availability of advanced treatments. Thus, this study aimed to assess determinants of survival outcomes among esophageal cancer patients in Kenya.
Methods
A retrospective cohort study was employed among 299 adult esophageal cancer patients. The data were collected using a data abstraction tool consisting of patients’ clinical characteristics and survival outcome measuring parameters. Statistical Package for the Social Sciences (SPSS) statistical software (version 20.0, IBM. USA) was used to analyze the data. The Kaplan–Meier and Cox regression analyses were used to determine the survival outcome and determinants of mortality, respectively.
Results
The mortality rate was 43.1%, and 11.1% of patients demonstrated distant metastases in the follow-up period. Despite treatment, 20.1% had progressed disease, and 13.0% did not respond to treatment. Radiotherapy (AHR: 3.3, 95% CI: 1.4−7.8, p = 0.007), chemotherapy (AHR: 3.9, 95% CI: 1.2−6.1, p = 0.020), and chemoradiation (AHR: 5.6, 95%CI: 1.6−10.2, p = 0.006) were the significant determinants of survival in advanced stage (III and and IV) patients.
Conclusions
There was a high mortality rate, disease progression, and nonresponse of esophageal cancer patients. Hence, it is essential to improve the survival of patients through early detection and timely initiation of the available treatment options.
Key points
There was a high mortality rate, disease progression, and nonresponse of esophageal cancer patients. Radiotherapy, chemotherapy, and chemoradiation were the significant determinants of survival in the advanced stage (III and and IV). Esophagectomy was the only treatment modality with a statistically significant effect on the survival outcomes of early-stage patients (I and II). Therefore, this study gave direction about the essence of early detection and timely initiation of the available treatment options to improve survival.
1 INTRODUCTION
Globally, esophageal cancer is the seventh leading type of cancer, with the highest incidence in Asian countries.1 In addition, it remains a significant cause of mortality and morbidity worldwide.2, 3 The incidence and mortality of esophageal cancer are high in African countries, with higher predominance in males due to the high prevalence of tobacco and alcohol consumption.4 A previous systematic review showed that morbidity of esophageal cancer was increasing at an alarming rate in the Sub-Saharan African regions with uneven geographical distribution.5
This disease has two major histological subtypes: squamous cell carcinoma and adenocarcinoma, with a global predominance of squamous cell carcinoma.6, 7 The risk of this cancer is significantly associated with consuming tobacco, alcohol, hot tea, and processed meat.4, 8
Most patients are currently diagnosed at a late stage with local or distant metastasis. Besides, many therapies do not confer satisfying survival benefits compared to other cancer populations.9 Despite improvements in managing esophageal cancer patients, the general outcome remains very poor.4, 10 However, a previous systematic review in Africa revealed a slightly improved survival with esophagectomy and chemoradiation therapy.4 Postoperative complications are the major cause of mortality associated with esophageal cancers.11 The 5-year overall survival rate is minimal, with the lowest cure possibilities.12-14 Despite the availability of several treatment options, such as surgery, chemotherapy, radiation therapy, and targeted therapy, the prognosis is still poor. Hence, achieving the desired goal of treatment remains challenging.15 Thus, this study aimed to assess the determinants of survival outcomes among esophageal cancer patients at Kenyatta National Hospital (KNH).
2 METHODS
2.1 Study design
A retrospective cohort study was employed at the Oncology Department of KNH. The hospital is the premier teaching and referral facility in Kenya, and the data were collected from September 2021 to January 2022.
2.2 Target population
This study targeted all medical records of adult patients with a confirmed diagnosis of esophageal cancers from 2016 to 2020.
2.3 Eligibility criteria
All medical records of adult patients (≥18 years) with a confirmed diagnosis of esophageal cancer from 2016 to 2020 with a complete medical record of diagnosis, stage of cancer, and treatment regimen were included in the study.
2.4 Sample size determination
Yamane's formula was employed to determine the sample size.16 In 2016–2020, around 849 esophageal cancer patients were treated in the study setting. As a result, the final sample size was 299 esophageal cancer patient files with a 10% contingency to cater for incomplete medical records.
2.5 Sampling techniques
A simple random sampling technique was employed to select the medical records of the patients. The list of all esophageal cancer patients who had been treated in 2016–2020 was generated by the Health Information Department of KNH. The researcher then screened the medical records to assess their eligibility using the established criteria. Patient identification numbers were listed on paper, folded, and put in a basket. Following meticulous shuffling, the researcher chose the patient identification numbers by lottery method until the required sample size was attained. Patient records with the selected identification numbers were included in the final study.
2.6 Data collection techniques and research instruments
A structured abstraction tool was used for the data collection. During the data abstraction process, the researcher perused the records and indicated all the variables of interest on the tool. They included the sociodemographic, clinical characteristics, histological types of cancer, time to death, or last follow-up period. Besides the time interval from the date of primary cancer diagnosis to the first radiographic metastasis, the time interval from the date of the first radiographic metastasis to the date of cancer related-death or last follow-up was also recorded. The response status of the patients (complete, partial, progression, nonresponse) was determined using the documented interval scan of the tumor.
2.7 Pretest study
Before beginning the actual study, a pretest was performed on 5% of the sample size to ensure the validity of the data collection instrument. Then, all necessary changes were made to the data collection instrument before executing the actual study.
2.8 Data analysis
SPSS statistical software (version 20.0, IBM. USA) was used to enter, clean, and analyze the data. The study variables were summarized using percent, frequency, mean, and standard deviation (SD). The Kaplan-Meier analysis was used to estimate the survival outcome. The log-rank test was used to compute the differences in survival probability across different treatment regimens. The potential predictors of mortality were identified using bivariate and multivariate Cox regression analysis. The outcome was considered statistically significant when p ≤ 0.05.
3 RESULTS
3.1 Socio-demographic characteristics of the study participants
A total of 299 eligible esophageal cancer patients were involved in the study. The median age of the study participants was 58.0 ± 12.7 years (range 18.0–93.0 years). Most study participants were males (178, 59.5%) and self-employed (145, 48.5%) (Table 1).
| Variable | n (%) |
|---|---|
| Age | |
| <60 years | 155 (51.8) |
| ≥60 years | 144 (48.2) |
| Gender | |
| Male | 178 (59.5) |
| Female | 121 (40.5) |
| Marital status | |
| Single | 22 (7.4) |
| Married | 250 (83.6) |
| Divorced | 7 (2.3) |
| Widowed | 20 (6.7) |
| Educational status | |
| Primary | 177 (59.2) |
| Secondary | 103 (34.4) |
| Tertiary | 12 (4.0) |
| Illiterate | 7 (2.3) |
| Occupational status | |
| Housewife | 18 (6.0) |
| Government employee | 18 (6.0) |
| Unemployed/Retired | 43 (14.4) |
| Selfemployed | 145 (48.5) |
| Family history of cancer | |
| No | 298 (99.7) |
| Yes | 1 (0.3) |
3.2 Clinical characteristics of the study participants
The current study showed squamous cell carcinoma was the most common histological type of esophageal cancer (281, 94.0%). At the time of diagnosis, most patients were in stages II and III of the disease (247, 82.7%), and 38 (12.7%) were in stage IV. In addition, the lung and liver were the most common sites of distant metastasis. Comorbid diseases were present in 124 (41.5%) participants. The most common coexisting conditions were hypertension, pneumonia, anemia, and retroviral disease (Table 2).
| Variables | n (%) |
|---|---|
| Histological type of cancer | |
| Adenocarcinoma | 18 (6.0) |
| Squamous cell carcinoma | 281 (94.0) |
| Stage of cancer | |
| Stage I | 14 (4.7) |
| Stage II | 141 (47.2) |
| Stage III | 106 (35.5) |
| Stage IV | 38 (12.7) |
| Comorbidity | |
| Present | 124 (41.5) |
| Absent | 175 (58.5) |
| Number of comorbidities | |
| One | 70 (23.4) |
| Two | 36 (12.0) |
| ≥Three | 18 (6.0) |
| Type of comorbidity | |
| Hypertension | 31 (10.4) |
| Pneumonia | 22 (7.4) |
| Anemia | 21 (7.0) |
| Retroviral disease | 21 (7.0) |
| Acute kidney injury | 17 (5.7) |
| Diabetes mellitus | 13 (4.3) |
| Sepsis | 8 (2.7) |
| Upper gastrointestinal bleeding | 8 (2.7) |
| Benign prostatic hyperplasia | 7 (2.3) |
| Deep vein thrombosis | 6 (2.0) |
| Chronic kidney disease | 5 (1.7) |
| Tuberculosis | 5 (1.7) |
| Pulmonary embolism | 5 (1.7) |
| Gastric outlet obstruction | 4 (1.3) |
| Upper airway obstruction | 4 (1.3) |
| Obstructive jaundice | 3 (1.0) |
| Chronic heart failure | 3 (1.0) |
| Hepatitis | 2 (0.7) |
| Esophageal candidiasis | 2 (0.7) |
| Cor pulmonale | 2 (0.7) |
| Epilepsy | 2 (0.7) |
| Gastroesophageal reflux disease | 1 (0.3) |
| Arthritis | 1 (0.3) |
| Stroke | 1 (0.3) |
| Hypothyroidism | 1 (0.3) |
| Atelectasis | 1 (0.3) |
| Distance metastasis at diagnosis | 38 (12.7) |
| Lung | 23 (7.7) |
| Liver | 9 (3.0) |
| Brain | 2 (0.7) |
| Pancreas | 1 (0.3) |
| Bone | 1 (0.3) |
| Liver, spleen, and lung | 1 (0.3) |
| Liver and lung | 1 (0.3) |
3.3 Treatment regimens of the study participants
In terms of management, esophagectomy (192, 64.2%), radiotherapy (107, 35.8%), and chemotherapy (69, 23.1%) were the most frequently used treatment modalities. Of these chemotherapy-treated patients, 26 (8.7%) were treated with carboplatin and paclitaxel regimens.
3.4 Survival outcomes of the study participants
During the follow-up period, 29 (11.1%) participants had signs of distant metastases. Metastases in the liver, lung, and brain were the most prevalent dissemination sites. The mortality rate was 43.1% (129), with censured outcomes in 170 patients. Sixty (20.1%) had progressed disease, and 39 (13.0%) were nonresponses despite treatment in the last follow-up period. Fourteen percent of patients (43) had a partial response, while 23 (7.7%) had a complete response. Five (1.7%) patients had unknown treatment outcomes in the follow-up period.
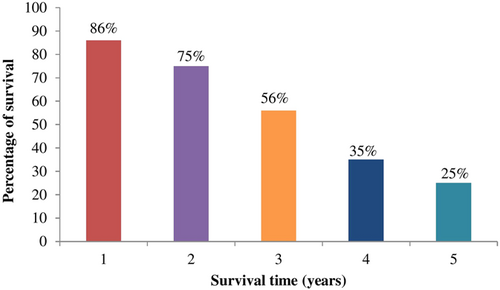
The 1- and 5-year survival rate of patients was 86% and 25%, respectively. Furthermore, the survival rate decreased from 2 to 5 years (Figure 1). The average survival time from diagnosis to the last follow-up or death (cancer-specific survival) was 10 months, whereas the mean survival time from diagnosis to the first radiological metastasis (metastasis-free survival) was 16.5 ± 4.2 months. Besides, the average survival time following metastasis was shorter (5.0 ± 0.7 months) than the average cancer-specific and metastasis-free survival time.
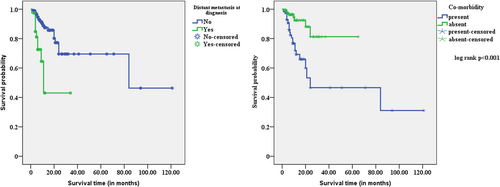
When comparing age groups, gender, stage of cancer, and histological type of cancer to their respective counterparts, the study found no statistically significant mean differences in survival estimates. However, the mean survival estimates of patients with comorbidity (55.9 ± 3.6 months) and distant metastasis at diagnosis (18.9 ± 3.5 months) were shorter than their counterparts. On the other hand, patients who underwent esophagectomy, radiotherapy, or chemoradiation had no differences in mean survival estimates compared to their counterparts. Compared to other treatment modalities, chemotherapy-treated esophageal cancer patients had the longest duration of mean survival estimates (68.3 months) (Table 3, Figure 2).
| Variables | Survival estimate (months) | Log-rank test (p-value) |
|---|---|---|
| Age | 0.088 | |
| <60 years | 87.3 ± 10.1 (67.5–107.1) | |
| ≥60 years | 41.7 ± 6.4 (29.9–54.4) | |
| Gender | 0.122 | |
| Male | 55.1 ± 4.6 (46.0–64.1) | |
| Female | 71.7 ± 10.4 (51.3–92.2) | |
| Comorbidity | <0.001* | |
| Present | 55.9 ± 3.6 (48.8–62.9) | |
| Absent | 57.6 ± 10.6 (36.7–78.4) | |
| Stage of cancer | 0.360 | |
| Early-stage (I and II) | 72.6 ± 11.8 (49.5–95.7) | |
| Advanced stage (III and IV) | 67.8 ± 7.0 (54.0–81.5) | |
| Histological type of cancer | 0.861 | |
| Adenocarcinoma | 53.1 ± 8.2 (37.1–69.1) | |
| Squamous cell carcinoma | 75.8 ± 9.2 (57.7–93.7) | |
| Distant metastasis at diagnosis | <0.001* | |
| Yes | 18.9 ± 3.5 (11.9–25.9) | |
| No | 80.3 ± 9.4 (61.7–98.9) | |
| Distant metastasis in the follow-up period | 0.421 | |
| Yes | 64.2 ± 5.7 (53.1–75.4) | |
| No | 86.9 ± 15.2 (57.3–116.7) | |
| Treatment regimen | ||
| Chemotherapy | 0.015* | |
| No | 57.1 ± 4.1 (49.1–65.1) | |
| Yes | 68.3 ± 10.1 (48.6–87.9) | |
| Esophagectomy | 0.402 | |
| No | 63.9 ± 12.5 (39.5–88.5) | |
| Yes | 70.3 ± 5.9 (58.6–81.9) | |
| Radiotherapy | 0.109 | |
| No | 75.4 ± 9.9 (55.9–94.9) | |
| Yes | 69.5 ± 5.9 (57.9–81.1) | |
| Chemoradiation | 0.149 | |
| No | 63.6 ± 5.6 (52.6–74.6) | |
| Yes | 94.1 ± 13.7 (67.2-120.9) | |
| Symptomatic management | 0.013* | |
| No | 79.5 ± 9.3 (61.3–97.6) | |
| Yes | 37.4 ± 10.7 (16.4–58.5) |
- Note: Data are presented as mean ± standard error (95% CI). CI, confidence interval.
- *p ≤ 0.05.
3.5 Determinants of survival outcomes
Advanced-stage (stage III and IV) patients with coexisting comorbidities had a 7.5-fold increased risk of mortality compared to patients without comorbidities (adjusted hazard ratio [AHR]: 7.5, 95% confidence interval [CI]: 2.2–12.0, p = 0.001).
Patients with advanced esophageal cancer who did not receive radiotherapy (AHR: 3.3, 95% CI: 1.4–7.8, p = 0.007), chemotherapy (AHR: 3.9, 95% CI: 1.2–6.1, p = 0.020) and chemoradiation (AHR: 5.6, 95% CI: 1.6–10.2, p = 0.006) had a statistically significant higher risk of dying compared to patients who received the respective treatment modalities. In the early stage disease (stages I and II), patients treated with esophagectomy had a lower risk of death (AHR: 1.9, 95% CI: 1.2–3.6, p = 0.049) than those without esophagectomy. Nonetheless, other treatment modalities had not shown a statistically significant effect on the survival outcomes of early-stage patients. Age, gender, and histological type of cancer were not statistically significant determinants of survival outcomes among early and advanced-stage esophageal cancer patients (Table 4).
| Categories | Variables | Bivariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| Crude hazard ratio (95% confidence interval [CI]) | p-value | Adjusted hazard ratio (95% CI) | p-value | ||
| Early stages (I and II) | |||||
| Histological type | Adenocarcinoma | 1.0 | 1.0 | ||
| Squamous cell carcinoma | 2.1 (1.2–3.4) | 0.577 | 2.3 (1.2–3.3) | 0.982 | |
| Gender | Male | 1.0 | 1.0 | ||
| Female | 3.1 (1.1–9.1) | 0.034* | 2.9 (0.9–9.1) | 0.059 | |
| Comorbidity | Absent | 1.0 | 1.0 | ||
| Present | 1.6 (0.9–2.7) | 0.083 | 1.7 (0.6–5.1) | 0.351 | |
| Age | <60 years | 1.0 | 1.0 | ||
| ≥60 years | 0.6 (0.4–1.1) | 0.623 | 2.3 (0.7–7.1) | 0.163 | |
| Radiotherapy | Yes | 1.0 | 1.0 | ||
| No | 1.3 (0.7–2.1) | 0.396 | 1.4 (0.7–2.6) | 0.311 | |
| Esophagectomy | Yes | 1.0 | 1.0 | ||
| No | 1.3 (0.8–2.2) | 0.262 | 1.9 (1.2–3.6) | 0.049* | |
| Chemotherapy | Yes | 1.0 | 1.0 | ||
| No | 1.5 (0.8–2.7) | 0.248 | 1.4 (0.7–2.7) | 0.342 | |
| Chemoradiation | Yes | 1.0 | 1.0 | ||
| No | 1.1 (0.5–2.3) | 0.838 | 2.1 (0.8–5.0) | 0.133 | |
| Advanced stages (III and IV) | |||||
| Histological type | Adenocarcinoma | 1.0 | 1.0 | ||
| Squamous cell carcinoma | 0.8 (0.2–3.4) | 0.731 | 1.2 (0.3–5.9) | 0.799 | |
| Gender | Male | 1.0 | 1.0 | ||
| Female | 0.9 (0.4–2.4) | 0.897 | 0.9 (0.3–2.5) | 0.886 | |
| Comorbidity | Absent | 1.0 | 1.0 | ||
| Present | 2.7 (1.5–4.6) | 0.001* | 7.5 (2.2–12) | 0.001* | |
| Age | <60 years | 1.0 | 1.0 | ||
| ≥60 years | 0.9 (0.5–1.3) | 0.485 | 0.9 (0.4–2.6) | 0.976 | |
| Radiotherapy | Yes | 1.0 | 1.0 | ||
| No | 1.5 (0.8–2.5) | 0.175 | 3.3 (1.4–7.8) | 0.007* | |
| Esophagectomy | Yes | 1.0 | 1.0 | ||
| No | 1.0 (0.6–1.6) | 0.906 | 2.8 (0.8–3.9) | 0.435 | |
| Chemotherapy | Yes | 1.0 | 1.0 | ||
| No | 2.6 (0.9–7.1) | 0.040* | 3.9 (1.2–6.1) | 0.020* | |
| Chemoradiation | Yes | 1.0 | 1.0 | ||
| No | 2.3 (0.8–6.4) | 0.104 | 5.6 (1.6–10.2) | 0.006* | |
- *p ≤ 0.05.
4 DISCUSSION
Despite the advancement in treatment, current treatment modalities have minimal survival advantages in esophageal cancer patients.9 However, there was a lack of comprehensive data about the survival outcomes among esophageal cancer patients. Therefore, this study highlights survival outcomes in these patients.
Most study participants had an advanced stage of the disease in our setting, which could probably be linked to late-onset symptoms, which can significantly affect the desired treatment outcomes. A recent study reveals that the lung and liver are the most common sites of distant metastasis, which agrees with the findings in our setting.17 Zhang et al.18 reported a poor prognosis among patients in the metastatic stage of esophageal cancer. Therefore, the higher rate of disease progression in our setting could be linked to the high metastasis rate at the diagnosis and follow-up period.
Previous studies reported a significant delay in diagnosing esophageal cancer in Kenya.19 Furthermore, delays in diagnosing and treating esophageal cancer result in worse survival outcomes.20 Therefore, early screening programs should be implemented nationally to improve survival and reduce the cost of treatment. Furthermore, educational programs about modifiable risk factors of esophageal cancer, such as cigarette smoking and alcohol consumption, should be strictly implemented to enhance public awareness.4, 21 This finding agrees with two studies that reported the predominance of advanced-stage diseases among esophageal cancer patients.22, 23 In our setting, 41.5% of esophageal cancer patients had coexisting comorbidities, which adversely impacted the survival of esophageal cancer patients.24-26 This high prevalence of existing comorbid conditions can probably be linked to the high mortality rate, disease progression, and nonresponse to treatment.
In the metastatic stage, chemotherapy (p = 0.037) was the significant treatment that improved survival. Kim et al.27 also reported that postoperative chemotherapy had improved survival outcomes in esophageal cancer patients. Hence, chemotherapy is highly recommended in advanced stages to improve the survival of these populations.
A previous systematic review showed a high mortality rate of esophageal cancer in Africa.4 Furthermore, another study also reported a higher 90-day postoperative mortality rate after esophagectomy in low and middle-income countries.28 The mortality rate of esophageal cancer patients was 43.1% in our setting, which is higher than findings from Japan (18.7%), the United States (8.9%), and South Korea (7.9%).29-31 This observation may be due to advanced healthcare services in these countries. In addition, due to the overwhelming infectious disease burden and resource constraints, cancer care is the least priority of healthcare services in sub-Saharan Africa, leading to high mortality rates due to cancer.32 Therefore, early screening awareness programs and equitable access to cancer treatment should be available to overcome the high mortality burden in esophageal cancer patients in Kenya.
The 5-year survival rate was 25% among the study participants, which is lower than in other studies (83.4%).29 In addition, Iranian (31.2%) and Chinese studies (40.1%) reveal a higher rate of survival.33, 34 This disparity could probably be due to the difference in tumor grade, comorbidities, and age of the study participants, which can significantly impact survival.
The mean survival time was longer (10 months) than patients from Ethiopia (4 months). In addition, the overall 3-year survival rate (2.4%) was lower in the Ethiopian study compared to our setting (56%).35 Despite this, the mean survival time was longer in western countries than the African esophageal cancer patients.36-39 These disparities suggested that cancer care was not adequate in African countries. Therefore, for better outcomes in cancer care, an improvement in treatment and diagnostic facilities should be the utmost priority of the health care delivery system, especially in the sub-Saharan African setting.
Patients who underwent esophagectomy, radiotherapy, or chemoradiation had no differences in mean survival estimates compared to their counterparts. In contrast, an Ethiopian study revealed a better survival outcome, although most patients had low overall survival.35 In addition, a previous study shows a relatively low overall rate of survival, although surgically treated patients had a significant improvement in survival outcomes.40 In our setting, chemotherapy-treated patients had the longest duration of mean survival estimates compared to other treatment modalities suggesting possible priorities in eligible esophageal cancer patients.
Previous studies reported that age and high Charlson comorbidity score are significant predictors of mortality in esophageal cancer.31 Similarly, the present study showed that patients with comorbidity had a higher mortality risk in advanced-stage disease. The high mortality rate could also be attributed to complications arising from multiple comorbidities that the patients had, which complicate the management of esophageal cancer. Close monitoring should therefore be done among patients with comorbidities that are life-threatening. Previous systematic reports esophagectomy and chemoradiation were the best treatment approaches to improve survival in esophageal cancer patients in the African population.4 In our setting, chemotherapy, radiotherapy, and chemoradiation-treated patients had a lower mortality risk among advanced-stage patients.
Nonetheless, esophagectomy was the only significant determinant of survival in the early stage (stage I and andII) patients. Likewise, Hassen et al.35 reported chemotherapy, radiotherapy, and surgical treatment approach as significant predictors of survival outcomes in esophageal cancer patients. In addition, chemoradiation and surgically treated locally advanced esophageal cancer patients had long-term overall survival.41
In conclusion, there was a high mortality rate (43.1%), disease progression (20.1%), and nonresponse (13.0%) of esophageal cancer patients. Radiotherapy, chemotherapy, chemoradiation, and comorbidity were the significant determinants of survival in advanced-stage patients. Esophagectomy was the only significant determinant of survival in early-stage disease. Early screening awareness programs and equitable access to cancer treatment should be available to overcome the high mortality burden in esophageal cancer patients in Kenya.
AUTHOR CONTRIBUTIONS
Amsalu Degu, Peter N. Karimi, Sylvia A. Opanga, and David G. Nyamu were involved from the conception to the final manuscript preparation. All authors reviewed and approved the manuscript.
ACKNOWLEDGMENTS
The authors would like to acknowledge all the oncology staff members at Kenyatta National Hospital who assisted us in collecting the data for the study.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The Kenyatta National Hospital/the University of Nairobi Ethics and Research Committee approved the protocol (Approval No: P195/03/2021). Due to the retrospective nature of the study design, the Ethics committee waived informed written consent. Before collecting data from patients’ medical records, official permission was obtained from the Health Information Department of the hospital. To protect the patient's privacy, the names and addresses of the patients were not recorded on the data collection tool. Since the study was retrospective, we obtained participants’ informed consent waivers from the Ethics committee.
Open Research
DATA AVAILABILITY STATEMENT
The datasets in the current study can be obtained from the corresponding author of this project.




