Changes in body composition in relation to estimated glomerular filtration rate and physical activity in predialysis chronic kidney disease
Edited by Yi Cui
Abstract
Background
Early body composition changes, associated with physical inactivity and disease advancement are devastating for patient-related outcomes in predialysis chronic kidney disease (CKD), thus warranting a detailed analysis of body composition beyond conventional measures.
Methods
The study included 40 subjects diagnosed with CKD; recruited between January to May 2021. Body composition was measured using the multifrequency analyzer, InBody 770. International Physical Activity Questionnaire-Short Form was used to assess physical activity. Suitable statistical analyses were performed using SPSS 21.0.
Results
The mean age of the subjects was 58.68 ± 12.24 years. Sarcopenic obesity was prevalent in 62.5% of the subjects. Body mass index under identified obesity by 15% compared to percent body fat, especially in subjects with low muscle mass. The decline in a unit of estimated glomerular filtration rate (eGFR) significantly correlated with a decrease in weight (p = 0.02), body fat mass (p = 0.05), visceral fat area (p = 0.05), and phase angle (p = 0.01) with marginal changes in waist–hip ratio and extracellular water/total body water. The effect of physical activity on skeletal muscle mass was homogeneous between low and moderate levels, but significantly different from high activity level.
Conclusion
Changes in fat and fluid compartment were associated with eGFR decline, whereas higher physical activity positively affected body composition.
Highlights
- (1)
Significant findings of the study
- •
Body mass index underestimated obesity versus percent body fat, especially in subjects with low muscle mass.
- •
Estimated glomerular filtration rate decline is associated with the decline in fat compartment and phase angle, as well as overhydration.
- •
The effect on muscle mass and body fat varies between activity levels.
- •
- (2)
What this study adds
- •
In-depth data on body composition status and its association with disease progression in predialysis chronic kidney disease subjects.
- •
Degree of body composition abnormalities present in the population.
- •
Explores the effect of physical activity on body composition highlighting its importance.
- •
1 INTRODUCTION
The consequence of malnutrition arising from the interaction of a multitude of aberrations is devastating for the quality of life, physical functioning, and mortality of predialysis chronic kidney disease (CKD) patients. Globally, India occupies the third position in malnourished CKD patients with a pooled prevalence of 56.7%, among hemodialysis, peritoneal dialysis, and nondialysis CKD.1, 2 Even so, predialysis CKD patients are an understudied population as compared to hemodialysis in the Indian subcontinent.
Secondary protein deficiency caused by amino acid metabolism disturbances, toxin accumulation, appetite suppression due to increased circulating immune mediators, and metabolic acidosis, as well as poor nutrient intake, all contribute to hypoalbuminemia and muscle mass loss in CKD patients.3-7 Thus, a comprehensive evaluation of the subclinical malnutrition in all stages of CKD is the need of the hour to prevent the onset of apparent malnutrition and improve associated patient outcomes. In this regard, assessment of body composition beyond the metric of body mass index (BMI) is essential in CKD to evaluate the alterations associated with the disease processes. Despite the presence of superior methods, dual-energy X-ray absorptiometry (DXA) and single- or multi-frequency bioelectrical impedance analysis (BIA) are used in clinical practice, regardless of the possible interference of hydration in optimal measurement.8, 9 Due to the additional advantage of portability, affordability, and absence of radiation, BIA may be more useful than DXA in routine use.
In addition, physical activity has recently been investigated because of its favorable effects on overall health in individuals with CKD. Although several studies have explored the effects of exercise and increased physical activity on body compartments such as skeletal mass, strength improvement, and loss of fat mass, there is little to no evidence from the Indian region.1 Prevalent physical inactivity of the Indian population as compared to global estimates and recommended standards further strengthens the need for exploring the beneficial effects.10, 11 This study aimed to identify the body composition alterations in the regional predialysis population using BIA, their relation with estimated glomerular filtration rate (eGFR) decline, and the effects of physical activity on the parameters.
2 METHODS
2.1 Subjects
This was an exploratory study conducted from January to May 2021 in the Mysuru district of India. Male or female subjects above 18 years, with or without comorbidities such as diabetes, hypertension, and cardiovascular conditions in conjunction with a clinical diagnosis of CKD as referred by a Nephrologist, were considered for inclusion in the study. Exclusion criteria were as follows: dialysis regimen, renal transplant recipients, history of infection, trauma or major surgery within 1 month of recruitment, and other catabolic conditions such as cancer, chronic obstructive pulmonary disease, and cirrhosis. Patients with metallic implantations, amputees or physical disability, or inability to stand upright were also considered ineligible. Serum creatinine, blood pressure, and random blood sugar levels were noted from the medical records of the subjects. Jaffe's and GOD–POD (glucose oxidase–peroxidase) methods were used by the laboratories to test serum creatinine and random blood sugar levels, respectively, on nonfasting venous samples.
Demographic data, medical history, and serum creatinine levels were retrieved from the patient's medical records. CKD-Epidemiology Collaboration creatinine equation (2009) was used to calculate eGFR. Height was measured using a digital stadiometer, BSM 170. BMI was calculated by dividing weight in kg by height square in meters. The multifrequency bioelectrical impedance analyzer, InBody 770, was used to measure various body composition parameters. The stadiometer and body composition analyzer were from InBody Co., Ltd. The instrument had eight-point tactile electrodes through which bioelectrical impedance and reactance were measured at frequencies ranging from 1 to 1000 kHz at each of the five body segments: right arm, left arm, trunk, right leg, and left leg. The assessment was performed in the morning hours using the standard protocol, that is, in the upright position, after voiding urine and excrement to avoid interference with weight measurement and at least 2 h post breakfast to avoid interference with the food mass. The subjects were asked to remove all the accessories, socks, and jewelry before testing.
2.2 Statistical analysis
Baseline categorical data and medical history were reported as percentages. The Shapiro–Wilk's test for normality revealed a nonnormal distribution (p < 0.05) of the data with respect to categories of the disease. Hence, differences between groups of variables were compared by Mann–Whitney U test (p < 0.05). Whereas the results of the Shapiro–Wilk's test irrespective of the disease categories indicated a normal distribution of the variables, warranting parametric tests. Karl Pearson's coefficients of correlation were obtained between eGFR and body composition parameters. Further, the parameters with a significant correlation with eGFR were considered to examine the multivariate relationships using a multivariate linear regression with eGFR as the independent variable and the selected body composition parameters as dependent variables. It was also observed that there was no effect of age, gender, hypertension, diabetes, and cardiovascular disease on selected dependent parameters. The χ2 test for association was applied to examine the association between chronic kidney disease categories and physical activity levels. Multivariate analysis of variance (ANOVA) was performed to examine the effect of physical activity levels on body composition parameters. For the parameters with significant results, Duncan's homogeneous subsets were obtained between physical activity levels. All statistically significant values were compared with a 0.05 or 0.01 level of significance. The statistical analysis was performed using SPSS 21.0 (IBM Corp.).
2.3 Outcome variables
Obesity identification by BIA-derived percent body fat was ≥25% in men and ≥30% in women, respectively.12 A high waist-hip ratio concurrent with abdominal obesity was defined with the cut-off of 0.8 for men and 0.9 for women.13 Alternatively, BIA obtained visceral fat area >100 cm2 was identified as abdominal obesity.14 Low muscle mass was ascertained in men with skeletal muscle index <7.0 kg/m2 and <5.7 kg/m2 in women according to the 2019 consensus update of the Asian Working Group for Sarcopenia (AWGS).15 Sarcopenic obesity, as per the European Society for Clinical Nutrition and Metabolism (ESPEN) and the European Association for the Study of Obesity (EASO)16 criteria, includes a combination of obesity by percent body fat (PBF) as well as low muscle mass measured by means of BIA.
3 RESULTS
A total of 40 subjects; 29 males and 11 females were recruited for the study. The demographic and medical history of the subjects is elaborated in Table 1. For comparison, the subjects were divided into an early-stage (ES) CKD group with mild to moderately decreased renal function and a late-stage (LS) CKD group with moderate to severely decreased renal function using a cut-off of 30 ml/min/1.73 m2. The ES group consisted of Stages 2 and 3, whereas the LS group consisted of Stages 4 and 5 as per the Kidney Disease Improving Global Outcome guidelines.3 Both the groups comprised majorly of men with 82.4% of the subjects in the ES group and 60.9% in the LS group. Hypertension was the most common comorbidity, followed by diabetes and cardiovascular disease in both groups. Even so, levels of random blood sugar and blood pressure did not vary significantly between the ES group and the LS group.
| Characteristics | Early-stage CKD, n (%) | Late-stage CKD, n (%) |
|---|---|---|
| Male | 14 (82.4) | 15 (65.2) |
| Female | 3 (17.6) | 8 (34.8) |
| Diabetes | 11 (64.7) | 15 (65.2) |
| Hypertension | 13 (76.5) | 20 (87) |
| Cardiovascular disease | 8 (47.1) | 6 (26.1) |
- Abbreviation: CKD, chronic kidney disease.
Table 2 depicts the clinical and body composition parameters of the subjects. The mean age of the subjects was 58.68 ± 12.24 years. The mean eGFR levels of the ES and LS groups were 43 ± 12 and 16 ± 7 ml/min/1.73 m2, respectively. The waist-hip ratio was significantly lower in the LS group compared to the ES group (p = 0.00). The ratio of extracellular water/total body water (ECW/TBW) was significantly higher in the LS group compared to the ES group (p = 0.02), whereas the whole-body phase angle was significantly higher in the ES group compared to the LS group (p = 0.02). Of interest, although statistically insignificant, the remainder of the indices of fat and fat-free compartment exhibited a trend of higher values in the ES group relative to the LS group.
| Characteristics | Early-stage CKD (n = 17) | Late-stage CKD (n = 23) | |z| | p Value | ||
|---|---|---|---|---|---|---|
| Mean ± SD | Median (25th–75th) | Mean ± SD | Median (25th–75th) | |||
| Clinical parameters | ||||||
| Age (years) | 57.8 ± 10.5 | 58 (49–65) | 59.3 ± 13.6 | 64 (52–69) | 0.81 | 0.42 |
| Serum creatinine (mg/dl) | 1.7 ± 0.4 | 1.7 (1.6–1.9) | 4.3 ± 2.1 | 3.7 (2.6–6.3) | 5.3 | 0.00** |
| eGFR (ml/min/1.73 m2) | 43 ± 12 | 41 (37–45) | 16 ± 7 | 16 (10–20) | 5.4 | 0.00** |
| RBS (mg/dl) | 150.8 ± 77.7 | 123 (98.5–177) | 142.2 ± 52.8 | 127 (99–187) | 0.11 | 0.91 |
| SBP (mmHg) | 137.7 ± 14.5 | 136 (129.5–143.5) | 150 ± 30.1 | 142 (130–164) | 1.16 | 0.24 |
| DBP (mmHg) | 77.5 ± 9.1 | 77 (68.5–84.5) | 80.2 ± 30.3 | 78 (72–90) | 0.37 | 0.71 |
| Body composition variables | ||||||
| Height (cm) | 166 ± 9.4 | 164 (160.4–170.7) | 159.7 ± 10.2 | 160.4 (150.3–167.6) | 1.79 | 0.08 |
| Weight (kg) | 74.8 ± 11.4 | 74.5 (64.2–83.2) | 67.0 ± 13.2 | 66.6 (57.5–79.1) | 1.78 | 0.08 |
| BMI (kg/m2) | 27.2 ± 3.4 | 26.9 (25–30.2) | 26.3 ± 5.1 | 24.2 (22.9–29.4) | 0.94 | 0.35 |
| Protein (kg) | 9.3 ± 2.0 | 8.7 (8.1–8.9) | 8.3 ± 2.0 | 7.7 (7.0–9.9) | 1.49 | 0.14 |
| Minerals (kg) | 3.3 ± 0.8 | 3.1 (2.8–3.5) | 3.1 ± 0.9 | 2.9 (2.4–3.5) | 1.12 | 0.26 |
| SLM (kg) | 44.9 ± 9.5 | 42.5 (39.1–48.7) | 40.9 ± 10.2 | 38.3 (33.9–48.1) | 1.46 | 0.14 |
| FFM (kg) | 47.7 ± 10.2 | 44.9 (41.4–51.6) | 43.5 ± 10.9 | 40.6 (35.9–51.1) | 1.40 | 0.16 |
| SMM (kg) | 26 ± 6 | 24.1 (22.2–27.8) | 23.2 ± 6.1 | 21.1 (19.0–27.7) | 1.48 | 0.14 |
| BMC (kg) | 2.7 ± 0.7 | 2.5 (2.4–2.9) | 2.6 ± 0.8 | 2.4 (2.0–3.0) | 0.86 | 0.39 |
| FFMI (kg/m2) | 17.1 ± 1.7 | 16.8 (15.6–18.2) | 16.8 ± 2.5 | 16.1 (14.7–18.6) | 0.67 | 0.50 |
| FMI (kg/m2) | 10.1 ± 3.8 | 11.1 (6.0–11.8) | 9.5 ± 5.2 | 8.2 (5.1–12.6) | 0.45 | 0.65 |
| SMI (kg/m2) | 7.2 ± 0.9 | 7.1 (6.5–8) | 7.0 ± 1.3 | 6.8 (5.9–7.8) | 1.00 | 0.32 |
| BFM (kg) | 27.2 ± 9.4 | 29 (18.6–33.4) | 23.5 ± 11.6 | 18.8 (14.2–32.4) | 0.96 | 0.34 |
| PBF (%) | 36.1 ± 10.6 | 39 (24.6–43.2) | 34.3 ± 12.9 | 34.4 (22.7–46.3) | 0.68 | 0.49 |
| VFA (cm2) | 140 ± 53.3 | 153.1 (87.3–166.3) | 116.6 ± 59.5 | 105.4 (64–168.2) | 1.00 | 0.32 |
| Waist–hip ratio | 0.9 ± 0.1 | 0.91 (0.85–0.99) | 0.8 ± 0.1 | 0.83 (0.77–0.90) | 3.01 | 0.00** |
| ECW/TBW | 0.39 ± 0.01 | 0.39 (0.38–0.39) | 0.40 ± 0.01 | 0.40 (0.39–0.40) | 2.44 | 0.02* |
| BCM (kg) | 30.7 ± 6.6 | 28.7 (26.6–32.7) | 27.6 ± 6.6 | 25.4 (23–32.6) | 1.46 | 0.14 |
| WBPA (°) | 4.9 ± 0.6 | 5 (4.6–5.3) | 4.3 ± 0.9 | 4.2 (3.5–5.0) | 2.33 | 0.02* |
- Abbreviations: BCM, body cell mass; BFM, body fat mass; BMC, bone mineral content; BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; ECW/TBW, extracellular water/total body water; FFM, fat-free mass; FFMI, fat-free mass index; FMI, fat mass index; PBF, percent body fat; RBS, random blood sugar; SBP, systolic blood pressure; SLM, soft lean mass; SMI, skeletal mass index; SMM, skeletal muscle mass; VFA, visceral fat area; WBPA, whole-body phase angle.
- * p < 0.05;
- ** p < 0.01.
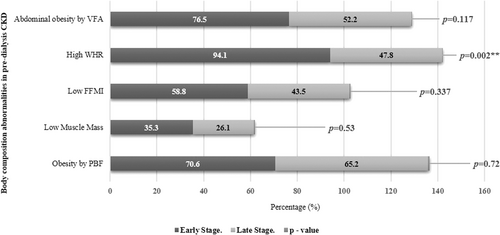
The prevalence of body composition abnormalities across the groups is shown in Figure 1. The incidence of a high waist-hip ratio was significantly greater in the ES group compared to the LS group (p = 0.00). Sarcopenic obesity was prevalent in 62.5% of the patients, whereas 30% of the patients had low muscle mass, according to AWGS criteria. Abdominal obesity was identified in 62.5% of subjects by visceral fat area cut-off, whereas WHR cut-off classified 67.5% of subjects. Likewise, obesity by PBF was identified in 67.5% of subjects, whereas BMI classified 52.5% of the subjects as obese, depicting under identification by 15%.
Correlation of eGFR with body composition parameters is established in Table 3. A significant positive correlation was observed between eGFR and weight (p = 0.02), body fat mass (p = 0.05), waist–hip ratio (p = 0.00), visceral fat area (p = 0.05), and phase angle (p = 0.01), whereas the ratio of ECW to TBW was significantly and negatively correlated with eGFR (p = 0.01).
| Variables | eGFR | |
|---|---|---|
| r | p Value | |
| Height (cm) | 0.203 | 0.21 |
| Weight (kg) | 0.361 | 0.02* |
| Body mass index (kg/m2) | 0.249 | 0.12 |
| Protein (kg) | 0.163 | 0.32 |
| Minerals (kg) | 0.039 | 0.81 |
| Soft lean mass (kg) | 0.133 | 0.41 |
| Fat-free mass (kg) | 0.125 | 0.44 |
| Skeletal muscle mass (kg) | 0.164 | 0.31 |
| Bone mineral content (kg) | 0.017 | 0.92 |
| Fat-free mass index (kg/m2) | 0.047 | 0.78 |
| Fat mass index (kg/m2) | 0.215 | 0.18 |
| Skeletal muscle index (kg/m2) | 0.107 | 0.51 |
| Body fat mass (kg) | 0.311 | 0.05* |
| Percent body fat (%) | 0.217 | 0.18 |
| Visceral fat area (cm2) | 0.307 | 0.05* |
| Waist–hip ratio | 0.457 | 0.00** |
| ECW/TBW | −0.388 | 0.01* |
| Body cell mass (kg) | 0.161 | 0.32 |
| WBPA (°) | 0.430 | 0.00** |
- Abbreviations: ECW/TBW, extracellular water/total body water; eGFR, estimated glomerular filtration rate; WBPA, whole-body phase angle.
- * p < 0.05;
- ** p < 0.01.
The parameters that correlated with eGFR at p < 0.05 in univariate analysis were further subjected to multivariate analysis, the results of which are presented in Table 4. For the decline in one unit of eGFR as measured in ml/min/1.73 m2, there was a decrease in weight by 0.28 kg (p = 0.02), body fat mass decreased by 0.20 kg (p = 0.05), visceral fat area decreased by 1.06 cm2 (p = 0.05) and phase angle decreased by 0.02° (p = 0.01). In addition, there was a minimal decrease in the waist-hip ratio (p = 0.00) while the ratio of ECW to TBW increased marginally (p = 0.01).
| Variables | Independent variable | B | SE | t | p Value | 95% Confidence interval |
|---|---|---|---|---|---|---|
| Weight (kg) | Intercept | 62.61 | 3.77 | 16.61 | 0.00** | 54.983–70.244 |
| eGFR | 0.28 | 0.12 | 2.38 | 0.02* | 0.042–0.518 | |
| ECW/TBW | Intercept | 0.40 | 0.00 | 120.13 | 0.00** | 0.395–0.409 |
| eGFR | 0.00 | 0.00 | −2.60 | 0.01* | 0.000–0.000 | |
| BFM (kg) | Intercept | 19.52 | 3.19 | 6.12 | 0.00** | 0.000–25.980 |
| eGFR | 0.20 | 0.10 | 2.02 | 0.05* | 0.000–0.402 | |
| WHR | Intercept | 0.80 | 0.03 | 28.92 | 0.00** | 0.000–0.859 |
| eGFR | 0.00 | 0.00 | 3.17 | 0.00** | 0.000–0.004 | |
| VFA (cm2) | Intercept | 97.37 | 17.05 | 5.71 | 0.00** | 0.000–131.897 |
| eGFR | 1.06 | 0.53 | 1.99 | 0.05* | 0.000–2.136 | |
| WBPA (°) | Intercept | 3.96 | 0.23 | 17.38 | 0.00** | 0.000–4.420 |
| eGFR | 0.02 | 0.01 | 2.93 | 0.00** | 0.000–0.035 |
- Abbreviations: BFM, body fat mass; ECW/TBW, extracellular water/total body water; eGFR, estimated glomerular filtration rate; VFA, visceral fat area; WBPA, whole-body phase angle; WHR, waist–hip ratio.
- * p < 0.05;
- ** p < 0.01.
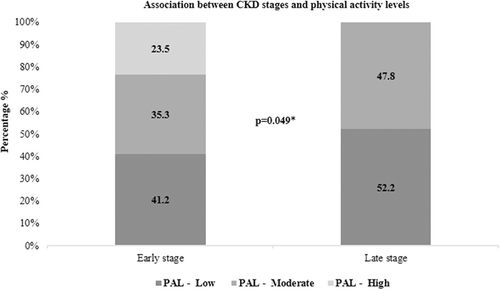
The relationship between eGFR and physical activity levels is depicted in Figure 2. χ2 analysis showed a statistically significant association between CKD disease stages and levels of physical activity (p = 0.05). Further, the influence of physical activity levels on body composition parameters is detailed in Table 5. Overall, no effect of physical activity level was observed on body composition parameters (p = 0.34). Contrarily, one-way ANOVA revealed a statistically significant difference between groups for height (p = 0.02), protein status (p = 0.01), percent body fat (p = 0.03), soft lean mass (p = 0.02), fat-free mass (p = 0.02), skeletal muscle mass (p = 0.01), and body cell mass (p = 0.01). Post hoc Duncan's homogeneous subset was applied to the body composition parameters, which was associated with physical activity levels at p < 0.05. The results of the homogeneous subset are represented in Table 6. It was observed that the effect of physical activity on height, protein status, soft lean mass, and skeletal muscle mass were homogeneous between low and moderate activity levels, but significantly differed from that of high activity levels. Whereas the effect on percent body fat, fat-free mass, and body cell mass was significantly different between low and high physical activity levels.
| Variables | High (n = 4) | Low (n = 19) | Moderate (n = 17) | F(2,37) | p Value | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| Height (cm) | 173.45 | 9.72 | 158.84 | 8.55 | 163.65 | 10.38 | 4.211 | 0.02* |
| Weight (kg) | 77.03 | 19.84 | 69.93 | 12.60 | 69.20 | 11.97 | 0.596 | 0.56 |
| Body mass index (kg/m2) | 25.30 | 4.52 | 27.72 | 4.81 | 25.81 | 3.87 | 1.052 | 0.36 |
| Protein (kg) | 11.10 | 2.12 | 8.00 | 1.51 | 9.02 | 2.15 | 4.898 | 0.01* |
| Minerals (kg) | 3.88 | 0.84 | 2.91 | 0.66 | 3.30 | 0.93 | 2.81 | 0.07 |
| Soft lean mass (kg) | 53.43 | 9.74 | 39.06 | 7.64 | 44.12 | 10.65 | 4.382 | 0.02* |
| Fat-free mass (kg) | 56.63 | 10.45 | 41.49 | 8.17 | 46.86 | 11.42 | 4.25 | 0.02* |
| Skeletal muscle mass (kg) | 31.38 | 6.46 | 22.11 | 4.56 | 25.23 | 6.41 | 4.903 | 0.01* |
| Bone mineral content (kg) | 3.22 | 0.73 | 2.42 | 0.56 | 2.73 | 0.79 | 2.555 | 0.09 |
| Fat-free mass index (kg/m2) | 18.65 | 1.53 | 16.28 | 1.57 | 17.26 | 2.63 | 2.48 | 0.10 |
| Fat mass index (kg/m2) | 6.63 | 3.47 | 11.43 | 4.90 | 8.54 | 3.93 | 3.04 | 0.06 |
| Skeletal mass index (kg/m2) | 7.93 | 0.67 | 6.67 | 0.86 | 7.36 | 1.38 | 3.055 | 0.06 |
| Body fat mass (kg) | 20.40 | 11.52 | 28.44 | 11.43 | 22.34 | 9.10 | 1.954 | 0.16 |
| Percent body fat (%) | 25.20 | 8.47 | 39.68 | 11.44 | 32.22 | 11.12 | 3.791 | 0.03* |
| Visceral fat area (cm2) | 97.53 | 55.21 | 147.95 | 58.36 | 109.41 | 50.62 | 2.833 | 0.07 |
| Waist–hip ratio | 0.92 | 0.10 | 0.90 | 0.12 | 0.85 | 0.07 | 1.468 | 0.24 |
| ECW/TBW | 0.39 | 0.01 | 0.40 | 0.01 | 0.39 | 0.01 | 0.891 | 0.42 |
| Body cell mass (kg) | 36.65 | 7.07 | 26.46 | 5.00 | 29.89 | 7.05 | 4.913 | 0.01* |
| WBPA (°) | 5.13 | 0.59 | 4.35 | 0.82 | 4.59 | 0.80 | 1.648 | 0.22 |
- Abbreviation: ECW/TBW, extracellular water/total body water; WBPA, whole-body phase angle.
- * p < 0.05.
| Variables | Response | n | Subset | |
|---|---|---|---|---|
| 1 | 2 | |||
| Height (cm) | Low | 19 | 158.836 | |
| Moderate | 17 | 163.652 | ||
| High | 4 | 173.450 | ||
| p Value | 0.32 | 1.00 | ||
| Protein (kg) | Low | 19 | 8.000 | |
| Moderate | 17 | 9.017 | ||
| High | 4 | 11.100 | ||
| p Value | 0.27 | 1.00 | ||
| Percent body fat (%) | Low | 4 | 25.200 | |
| Moderate | 17 | 32.223 | 32.223 | |
| High | 19 | 39.684 | ||
| p Value | 0.21 | 0.18 | ||
| Soft lean mass (kg) | Low | 19 | 39.058 | |
| Moderate | 17 | 44.118 | ||
| High | 4 | 53.425 | ||
| p Value | 0.27 | 1.00 | ||
| Fat-free mass (kg) | Low | 19 | 41.489 | |
| Moderate | 17 | 46.865 | 46.865 | |
| High | 4 | 56.625 | ||
| p Value | 0.28 | 0.05 | ||
| Skeletal muscle mass (kg) | Low | 19 | 22.105 | |
| Moderate | 17 | 25.229 | ||
| High | 4 | 31.375 | ||
| p Value | 0.26 | 1.00 | ||
| Body cell mass (kg) | Low | 19 | 2.421 | |
| Moderate | 17 | 2.728 | 2.728 | |
| High | 4 | 3.223 | ||
| p Value | 0.37 | 0.15 | ||
4 DISCUSSION
The current study elucidates body composition and examines the effects of eGFR decline and physical activity on the parameters. The population under study demonstrated body composition abnormalities with low muscle mass as well as higher body fat. In a study by Tyrovolas et al.17 the prevalence of sarcopenia was higher at 17.5% in older Indian adults compared to other countries, owing to the low physical activity levels. Low muscle mass was prevalent in 30% of the CKD patients in the current study. Contrastingly, in a study by Dubey et al.,18 the prevalence of low muscle mass using the similar cut-off of AWGS but the three-compartment DXA model instead of multifrequency BIA, was much higher at 69.1%. The lack of consensus in the functional definition, varied cut-points, and use of different methods to measure muscle mass is perhaps the basis of disparity regarding the prevalence of low muscle mass across studies even among the Indian subpopulation. Furthermore, physiological changes during aging, such as denervation and reinnervation resulting in muscle fiber-type grouping, hinder the definitive assessment of disease-related secondary sarcopenia.19
Obesity is described as an excess accumulation of body fat with health implications. BMI is a simple metric typically used to classify stages of underweight and obesity, although it does not demarcate the weight associated with muscle from the weight associated with fat.20 In the present study, BMI under identified obesity in 15% of the patients, whereas almost none of them were overidentified. The high prevalence of sarcopenia in this population seems to play a compensatory role for the increased fat percentage, thus contributing to the misclassification. In agreement with the above, 83% of the patients were classified under sarcopenic obesity using the ESPEN and EASO criteria.16 This indifferent diagnostic performance of BMI compared to percent body fat for obesity was also observed in a study by Sharma et al.21 The authors also reported a high prevalence of sarcopenia in the wrongly classified patients with an increase in the misclassification alongside eGFR decline.
Regarding the effect of eGFR decline on body composition parameters, it was observed that eGFR decline was associated with the decline in weight, fat compartment, phase angle, and accumulation of fluid, whereas no significant changes were observed in the muscle compartment. In accordance with this finding but using DXA instead of BIA, Zhou et al.22 reported a 0.26 ± 0.12 kg decrease in fat mass with every unit decrease in eGFR. Weight loss owing to a disease condition may increase the risk of mortality associated with a low BMI.23 A study by Ku et al.24 reported an increase in mortality upon dialysis initiation among CKD patients with an annual weight loss >5%. However, the study associated the weight loss with fat-free mass but not fat mass despite using BMI as a measure of weight, a better marker of fat mass than muscle mass. Underlining the paucity, studies exploring the association of CKD progression and mortality with fat loss, especially its distribution and various forms is essential in predialysis CKD subjects.
The measured phase angle reflects the health of the cells with respect to integrity and permeability of the cell membrane and has been studied as a plausible indicator of nutritional status and volume overload in CKD patients.25, 26 In the current study, a decline in eGFR was associated with a decrease in phase angle indicating a decrease in cell vitality as the disease progresses. In a study by Jha et al.,27 the phase angle of a healthy adult Indian population with a mean age of 39 ± 12 was found to be 6.6 ± 0.96 in men and 5.9 ± 0.94 in women, which was much greater than the observed values of phase angle in this study. However, the establishment of reference values is essential to properly assess the effects of varying levels on disease outcomes.
Conventionally, exercise was not recommended in CKD due to the argument that intense physical activity decreased the effective renal plasma flow concomitantly decreasing eGFR, possibly via involvement of sympathetic nervous activity and catecholamine substances.28 Contrastingly, recent studies have documented a neutral effect or a rather positive effect of exercise on eGFR.29, 30 When examining physical activity, it was found that vigorous physical activity had considerable effects on fat-free mass, that is, skeletal muscle mass, body cell mass, and body fat percentage as compared to low physical activity. Conforming to this finding, a study by Moon et al.,31 in Korean predialysis CKD patients, stated that physical activity obtained using the International Physical Activity Questionnaires (IPAQ) is noted to have protective effects against loss of muscle mass. Studies have also demonstrated an increase in O2 consumption due to exercise,32 which probably is a sequel to changes in metabolically active cell mass. Although in debate, subjective measure of physical activity such as IPAQ has been routinely used in the elderly and CKD population.33
In conclusion, low muscle mass is prevalent in predialysis CKD and needs careful consideration to improve quality of life. Physical activity is favorable in improving the metabolically active components of the body, such as skeletal muscle mass and body cell mass. Assessment of nutritional status using BIA provides an insight into the subclinical signs of malnutrition associated with the disease before it is apparent as BMI cannot be relied upon as an appropriate nutritional marker given the offsetting effect of low muscle mass against high-fat mass.
Findings from this study have to be interpreted in light of certain limitations and strengths. The study has a smaller sample size, hence there is a need to verify the findings in a larger sample. In addition, because of the exploratory nature of this study, causality cannot be established between eGFR decline and changes in body composition parameters. The plausibility of bias cannot be denied due to the subjective nature of the tool used to classify physical activity. Conversely, the use of BIA as opposed to conventional anthropometric measures provides detailed information about the nutritional status of the patients. Patients with predialysis CKD are an underinvestigated population in India. Thus, this study provides a well-founded premise for further investigations with larger samples to examine the extent of body composition alterations in the early stages of CKD and to establish valid clinical cut-offs for Indian patients.
AUTHOR CONTRIBUTIONS
Prathiksha R. Bhat was involved in formulating the design of the work as well as data acquisition, analysis, and interpretation. Asna Urooj was involved in the conception and design, provided critical intellectual input, and approved the manuscript for submission. Srinivas Nalloor was involved in data acquisition and provided valuable input to the design.
ACKNOWLEDGMENTS
The authors would like to thank the University of Mysore for the infrastructure provided and Mr. Santhosh CD for his assistance with the statistical analysis for this manuscript. This research was funded by University Grants Commission, New Delhi [UGC-Ref. No: 1593/(NET-DEC 2018)].
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The study conforms to the ethical standards as per the Declaration of Helsinki. Informed consent was obtained from all the participants’ prior inclusion. This study was approved by the Institutional Human Ethics Committee of the University of Mysore (IHEC-UOM No. 172/Ph.D/2019-20).
Open Research
DATA AVAILABILITY STATEMENT
Research data are not shared.