Pathophysiology and therapeutic advances in myeloma bone disease
Edited by: Yi Cui
Abstract
Bone disease is the most common complication in patients with multiple myeloma (MM), and it may lead to skeletal-related events (SREs) such as bone pain, pathological fractures, and spinal cord compression, which impair a patients' quality of life and survival. The pathogenesis of myeloma bone disease (MBD) involves disruption of bone reconstitution balance including excessive activation of osteoclasts, inhibition of osteoblasts, and participation of osteocytes and bone marrow stromal cells. Various factors, such as the receptor activator of nuclear factor-κB ligand (RANKL)/osteoprotegerin (OPG), dickkopf-1 (DKK-1), sclerostin, and activin-A, are involved in the development of MBD. Bisphosphonates and the anti-RANKL antibody denosumab are currently the main treatment options for MBD, delaying the onset of SREs. Denosumab is preferred in patients with MM and renal dysfunction. Although effective drugs have been approved, antimyeloma therapy is the most important method for controlling bone disease.
Highlights
-
Multiple myeloma is the leading cause of destructive bone disease.
-
Overactivation of osteoclasts and suppression of osteoblasts are involved in the pathogenesis of myeloma bone disease.
-
Bisphosphonates and denosumab are cornerstones to prevent skeletal-related events.
1 INTRODUCTION
Multiple myeloma (MM) is a malignant plasma cell disorder characterized by the overproduction of monoclonal immunoglobulins in the peripheral blood and urine with associated organ dysfunction. The main clinical manifestations of MM are hypercalcemia, renal insufficiency, anemia, and osteolytic bone destruction, abbreviated as CRAB. Myeloma bone disease (MBD) refers to all skeletal-related events (SREs), such as bone pain, pathological fractures, and spinal cord compression, and is one of the most common complications due to an imbalance between osteoclasts and osteoblasts. Bone lesions are present in more than 80% of patients with MM at the onset of the disease and are even more prevalent in refractory relapsed stages.1-3 Approximately 60% of patients develop pathological fractures during the course of the disease.4 The severity of MBD is positively correlated with tumor load and disease status, and patients with pathological fractures are at a significantly higher risk of mortality.5 MBD impairs the quality of life and survival. Therefore, focusing on the mechanisms of MBD and effective agents will help improve the prognosis.
2 NORMAL BONE REMODELING
In the bone marrow microenvironment, osteoclasts and osteoblasts, which are core components of bone reconstruction, account for less than 10% of all cells, while osteocytes account for 90% to 95%.6 These three types of cells maintain bone mineral density in the normal physiological state by participating in bone formation and bone resorption.
Osteoclasts are multinucleated cells that originate from the monocyte–macrophage hematopoietic cell lineage. Under the stimulation of two cytokines, monocyte/macrophage colony-stimulating factor and receptor activation of nuclear factor-κB (NF-κB) ligand (RANKL), progenitor cells differentiate into mature osteoclasts, which participate in the bone resorption process.7 Osteoblasts are derived from bone marrow stromal cells (BMSCs). Through collagen deposition, osteoid formation, and mineralization, osteoblasts are involved in new bone formation.8, 9 Osteocytes, transformed from osteoblasts, can be involved in inducing osteoclast activation by secreting various cytokines such as osteoprotegerin (OPG), RANKL, Dickkopf-1 (DKK-1), and others.6 Normal bone remodeling involves the removal of damaged bone by osteoclasts (i.e., bone resorption) and the formation of new bone by osteoblasts, wherein the two processes are in balance with each other. However, in patients with MM, this balance is disrupted.
3 PATHOGENESIS OF MBD
3.1 Activation of osteoclasts
One of the core pathophysiological mechanisms of MBD is the significant activation of osteoclasts, which results in a marked imbalance in bone remodeling. Myeloma cells initiate the stimulation of osteoclasts through various cytokines, thereby promoting bone resorption (Figure 1).
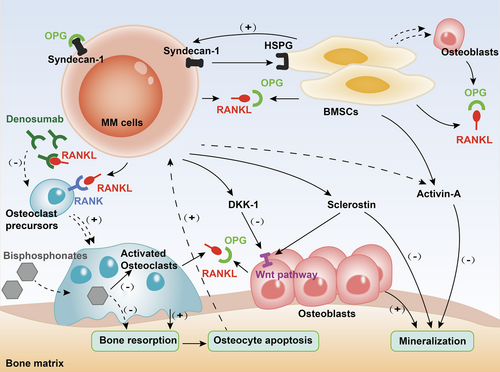
3.1.1 The RANK/RANKL/OPG pathway
Receptor activator of NF-κB (RANK) is a member of the tumor necrosis factor receptor family and is expressed on the surface of osteoclast precursors. RANKL is produced by BMSCs, osteocytes, and osteoblasts. It is also overexpressed in myeloma cells.10, 11 Osteoclast maturation is enhanced through the integration of RANKL with RANK.
OPG, a soluble decoy receptor for RANKL, is produced by osteoblasts and BMSCs. It maintains a delicate balance during bone remodeling by inhibiting osteoclast production and activation via competitive binding to RANKL.
Researchers have proven that through intercellular contacts between MM cells and BMSCs, OPG expression was significantly suppressed.12, 13 Without OPG, destruction of the bone cortex and trabeculae and severe osteoporosis were observed.14 After OPG was administered, the number of osteolytic lesions in the vertebrae and long bones was reduced, and the bone mineral density improved.11 In addition, the RANKL/OPG ratio was significantly higher in MM patients than in healthy controls, which could predict patients’ survival.15, 16
3.1.2 Syndecan-1 (CD138)
Syndecan-1 (CD138) molecule is expressed on the surface of normal plasma cells and myeloma cells. Syndecan-1 synergizes with the heparan sulfate proteoglycan expressed by BMSCs, allowing normal plasma cells and myeloma cells to enter the bone marrow niche and proliferate. When suppressed, marked inhibition of myeloma cell growth is observed.17 In MBD, syndecan-1 binds, internalizes, and degrades the RANKL antagonist, OPG, thereby promoting RANKL-mediated osteoclast activity and osteolysis.18 In addition, through upregulation of the expression of RANKL, soluble syndecan-1 reinforces osteoclastogenesis.19
3.1.3 Activin-A
Activin-A, a member of the transforming growth factor-β superfamily, is a multifunctional glycoprotein that binds to transmembrane receptors for signaling. Activin-A can activate the NF-κB pathway by inducing RANK expression, thereby promoting osteoclast differentiation.20 Several studies have confirmed that activin-A levels are significantly higher in patients with MM than in those with monoclonal gammopathy of undetermined significance (MGUS) or healthy controls. Elevation in his activin-A levels was strongly correlated with increased bone resorption biomarkers and extensive bone disease (a pathological fracture or more than three lytic lesions).21 Moreover, activin-A also inhibits osteoblast-mediated mineralization process, slowing bone formation in MM.22
3.2 Inhibition of osteoblasts
In addition to overactivation of bone resorption, suppression of osteoblast-mediated bone formation causes deterioration in bone remodeling (Figure 1). In contrast with bone metastases in solid tumors, such as prostate or breast cancer, osteogenesis is completely inhibited in MBD.23 Therefore, alkaline phosphatase (ALP) elevation is rarely seen in MM patients without comorbidities.24
3.2.1 Wnt pathway and DKK-1
In MBD, the classic (β-linked protein-dependent) Wnt signaling pathway is regulated by factors such as Dickkpof-1 (DKK-1, secreted frizzled-related proteins). DKK-1 is produced by osteoblasts, BMSCs, and myeloma cells. DKK-1 inhibits the signaling pathway by competitively binding to the receptors on the Wnt pathway, thereby suppressing osteoblast activity and new bone formation.25-27 Among newly diagnosed MM patients, those with bone lesions detected by imaging had significantly higher DKK-1 expression at both the gene and protein levels than controls without bone lesions, and this high-level DKK-1 could inhibit osteoblast differentiation.28
3.2.2 Sclerostin
Sclerostin is produced by both osteocytes and MM cells. One of its mechanisms of action involves binding to low-density lipoprotein receptor-related protein on the surface of the osteoblast cells, inhibiting the classical Wnt signaling pathway.29 In addition, sclerostin inhibits the binding of the bone morphogenetic proteins (BMPs) to ligands, such that osteoblast-mediated demineralization involving BMPs is downregulated and bone formation is inhibited.30
In a coculture system of BMSCs and MM cells secreting sclerostin, maturation and differentiation of osteoblasts were significantly inhibited.31 Patients with MM have significantly higher levels of sclerostin than those with MGUS, which is associated with lower osteoblast function and shorter survival.32, 33 Researchers also confirmed that sclerostin started to increase 4 months before relapse and was higher than that at complete response.34
3.3 Participation of osteocytes
Osteocytes are involved in MBD. In the normal bone marrow, osteocytes regulate the progress of bone remodeling by expressing RANKL, sclerostin, and OPG. Fewer viable osteocytes and more deaths were observed in bone biopsies of patients with MM than those in biopsies of controls. Among the patients, those with osteolytic lesions had significantly fewer viable osteocytes than those without skeletal involvement.35 Besides, apoptosis of osteocytes promoted the development and progression of MBD by altering the bone marrow microenvironment and supporting the homing and development of myeloma cells.36
3.4 Interactions with BMSCs
BMSCs play an important role in the function of the bone marrow microenvironment. BMSCs produce various cytokines to promote the growth of myeloma cells, forming a vicious circle. An increase in BMSCs has been observed in MM patients, which is in line with the MM tumor burden, accompanied by a decrease in osteoblasts.37 Through the interaction with MM cells, BMSCs show abnormal epigenetic aberrancies, thus impairing their ability to differentiate into osteoblasts, and targeting therapy has proved to be efficient in reversing MBD.38
4 TREATMENT OF MBD
4.1 Antimyeloma therapy
Antimyeloma therapy plays an essential role in controlling MBD progression. Some of the current preclinical studies suggest that bortezomib may be involved in mediating bone remodeling and that some osteogenic metabolites, such as ALP, increase after treatment with bortezomib.39, 40 Carfilzomib shows antibone resorption activity and promotes new bone formation.41, 42 Immunomodulatory drugs (IMiDs) are the cornerstone antimyeloma agents. However, some studies have demonstrated that IMiDs inhibit osteoblast activity, which is clinically manifested as a decrease in ALP and bone mineralization. Therefore, attention should be paid to the potential effects of IMiDs on bone metabolism.43
4.2 Bisphosphonates
Bisphosphonates are the primary treatment for MBD, as recommended by the authoritative guidelines. Common bisphosphonates, including zoledronate, pamidronate, and clodronate, are pyrophosphate analogs that bind to exposed hydroxyapatite and are absorbed by osteoclasts, delaying osteoclast recruitment and maturation and inducing osteoclast apoptosis by inhibiting farnesyl diphosphate synthase.44-46
The effect of bisphosphonates in reducing the incidence of SREs in patients with myeloma was reported in 1992. Lahtinen et al.47 demonstrated that patients with MM receiving clodronate had delayed progression of osteolytic lesions and a significant reduction in bone pain compared with those receiving placebo. However, there was no significant difference in the overall survival.48 Clinical trials showed comparable results with pamidronate and clodronate.49 Compared with clodronate and pamidronate, zoledronic acid showed superior or noninferior anti-MBD efficacy in several Phase III and IV trials. In addition, patients on zoledronic acid had increased median survival time and reduced mortality.50, 51 In contrast, the third-generation agent ibandronate failed to delay the development of SREs or improve survival.52 Recently, a meta-analysis including 7293 patients in 24 randomized controlled trials generalized the benefits of bisphosphonates compared with placebo or no treatment. Among these, zoledronic acid prevailed in preventing SREs, reducing pathological fractures and bone pain, and showed evidence of benefit for overall survival.53
Toxicity associated with bisphosphonate therapy includes osteonecrosis of the jaw (ONJ) and renal impairment.53, 54 Not all bisphosphonates are recommended for patients with creatinine clearance less than 30 ml/min. The dose of BPs should be reduced in patients with mild renal dysfunction. In addition, invasive dental procedures and poor oral hygiene are major risk factors for ONJ in patients treated with bisphosphonates.55 It is possible that the bone lesion may heal after drug discontinuation, but ONJ may recur with re-exposure to bisphosphonates.56
4.3 Denosumab
Denosumab is a human-derived monoclonal antibody that specifically targets RANKL, thereby inhibiting osteoclast activation and maturation. In 2011, Henry et al.57 reported 1776 patients with bone metastases or MM in a randomized controlled study and concluded that denosumab was not inferior to zoledronic acid in delaying time to first SREs. Although the survival analysis of a subgroup of MM patients favored zoledronic acid, the conclusion was not reliable due to unbalanced baseline characteristics in the two treatment groups.58 In 2018, another randomized, double-blind, controlled Phase III trial, which enrolled 1718 patients with MM, showed that denosumab did not differ from zoledronate in delaying first-onset SREs. In addition, patients receiving denosumab had a 10.7-month longer median progression-free survival than patients receiving zoledronic acid (46.1 vs. 35.4 months; hazard ratio: 0.82; 95% confidence interval: [0.68–0.99], p = 0.036).59
As denosumab is not eliminated via the kidney, it is not contraindicated in patients with renal insufficiency. Further, the incidence of ONJ (4%) in patients receiving denosumab did not differ from that in patients receiving zoledronic acid (3%). Although the rate of all-grade hypocalcemia was higher in the denosumab group (17% vs. 12%), serious hypocalcemia was around 1% in both groups. Therefore, calcium supplementation should be suggested during denosumab administration, and invasive oral manipulation should be avoided.
Some studies have concluded that treatment of anti-MBD alters the tumor microenvironment, which translates into indirect antimyeloma effects.60 Therefore, denosumab may affect myeloma cell growth and dormancy through the RANKL-mediated pathway.61 The potential antimyeloma effect of denosumab has received attention, but needs to be confirmed by further evidence in more trials.
4.4 Antiactivator A treatment
Activin-A promotes the development of MBD as it is an activator of osteoclasts and an inhibitor of osteoblasts. Sotatercept is a soluble ligand of the recombinant activin receptor type IIA fused to a human-derived immunoglobulin fragment that disrupts the cascade reaction involving activin-A. In a Phase II clinical trial, Abdulkadyrov et al.62 demonstrated enhanced bone mineral density and elevated ALP level after combined treatment with sotatercept with melphalan, prednisolone, and thalidomide regimen in MM patients. In addition, elevated hemoglobin levels were observed in all sotatercept dose groups, which was the main dose-limiting toxicity.63
4.5 Anti-DKK-1 therapy
Anti-DKK-1 antibodies antagonize the inhibition of osteoblast function by DKK-1. In animal studies, DKK-1 antibodies improved bone density and reduced osteolytic lesions in myeloma-bearing mice.64, 65 BHQ880, a DKK-1-neutralizing antibody, removed suppression of bone formation and showed potential therapeutic effects on myeloma in vivo.66 In Phase I clinical trial, BHQ880 combined with zoledronic acid in patients with refractory or relapsed MM demonstrated increased bone mineral density in the spine and hip.67 Although no recent data have been updated, the potential clinical application of anti-DKK-1 antibodies is still of interest.
4.6 Antisclerostin therapy
Sclerostin is produced by osteocytes and induces apoptosis of mature osteoblasts. Romosozumab, a humanized antibody against sclerostin, is approved for the prevention of osteoporosis in postmenopausal women at a high risk of fractures. Currently, in MM animal studies, antisclerostin treatment delays myeloma-induced bone loss, reduces osteolytic bone lesions, and increases fracture resistance.68 However, the application of romosozumab in MM remains to be explored.
5 CONCLUSION
Bone disease does not develop without the proliferation of myeloma cells. Although novel agents have remarkably improved the response and survival in patients with MM, bone disease is still an essential complication that impairs the patients’ quality of life and clinical outcomes. Advances in genetic or molecular mechanisms regarding the pathogenesis of MBD have translated into more bone-modifying therapies. Among them, bisphosphonates demonstrate a significant effect in terms of preventing SREs and are considered as the “cornerstone” front-line treatment. Denosumab, which targets the main pathway of RANKL/RANK/OPG, demonstrates comparable efficacy to zoledronic acid for suppressing osteoclasts and stimulating osteoblasts. It is safer in renal failure patients treated with denosumab than in those treated with bisphosphonates, while other toxicities are similar, such as hypocalcemia and ONJ. The antimyeloma effects of bone-modifying agents require further exploration.
AUTHOR CONTRIBUTIONS
Zhuang Junling: Conceptualization; writing—review; editing; funding acquisition. Zhang Fujing: Writing—original draft; visualization.
ACKNOWLEDGMENT
Capital Health Research and Development of Special Fund 2022-2-4013.
CONFLICT OF INTEREST
The authors declare no conflicts of interest. Professor Junling Zhuang is a member of Chronic Diseases and Translational Medicine editorial board and is not involved in the peer review process of this article.
ETHICS STATEMENT
This is a review and we obey all the Wiley's Publication Ethics Guidelines.
Open Research
DATA AVAILABILITY STATEMENT
This is a review, and all the data mentioned are from the original articles. These are available in a public repository. DOIs of the references are listed.