Translational Medicine in Alzheimer's Disease: The Journey of Donanemab From Discovery to Clinical Application
ABSTRACT
Substantial research has been conducted to identify an efficient treatment for Alzheimer's disease (AD). Existing treatments, including cholinesterase inhibitors and N-methyl D-aspartate (NMDA) receptor antagonists, do not reverse or slow the disease course but only treat its manifestations. This limitation has brought attention to the need for treatments that modify the amyloid-beta (Aβ) and tau pathology of the disease. One recent advancement in AD treatment is donanemab, a monoclonal antibody intended to clear Aβ plaques in the brain. It targets pyroglutamyl(3)-Aβ protein (3–42) to remove Aβ deposits and alter the disease course. This review explores the timeline of donanemab use from discovery to clinical use. The pharmacodynamics and pharmacokinetics of the drug are discussed along with typical and suboptimal preclinical and clinical trial results in terms of efficacy, safety, and tolerability. Thus, donanemab is more effective than donepezil and rivastigmine in removing plaques and improving cognition. At the same time, it is not devoid of safety concerns that are typical of the majority of amyloid-targeted medicines. The control to end the treatment after plaque cleaning is a unique selling point for some patients, making it more attractive. The innovation and development of donanemab from research to clinical practice are a clear representation of the role of the field of translational medicine in the practical application of new knowledge in the treatment of AD.
Summary
-
Donanemab is a monoclonal antibody that targets amyloid-beta plaques in Alzheimer's disease (AD).
-
Donanemab enables microglial activation and clearance of amyloid plaques from neurons.
-
Donanemab is designed for patients with early symptomatic AD, including MCI due to AD and mild AD dementia.
-
Preclinical and clinical trials demonstrated efficacy in reducing plaques and slowing cognitive decline.
1 Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disorder that predominantly affects the elderly and is characterized by memory loss, cognitive decline, and behavioral changes [1, 2]. Observed by participants with AD, patients have a four times higher mortality rate than those without dementia, with an average life expectancy of 6 years from the time of diagnosis. The data also reveal that, by the year 2024, AD will affect approximately 6 million people in America who are 65 years of age and older [3]. The disease is characterized by the formation of amyloid-β (Aβ) plaques and neurofibrillary tangles, which comprise hyperphosphorylated tau protein that adversely affects functioning neurons and causes cell death [4, 5].
Existing therapies for AD, including cholinesterase inhibitors, such as Donepezil, Rivastigmine, and Galantamine, offer only moderate efficacy and provide symptomatic relief without modifying the disease itself [6, 7]. Their benefits are short-lived, typically lasting 6–12 months, and they have side effects, such as nausea, vomiting, diarrhea, and fatigue, with limited effectiveness in the advanced stages of the disease. Similarly, inhibitors and N-methyl D-aspartate (NMDA) receptor antagonists like Memantine also provide symptomatic relief with short-term benefits and side effects like dizziness, headache, and confusion but are less effective in cases of mild cognitive impairment [8]. Anti-amyloid antibodies, such as Lecanemab (Leqembi [Eisai R&D Management Co. Ltd., Nutley, NJ]) [9] and Aducanumab (Aduhelm [Biogen, Cambridge, MA]) [10], show variable efficacy, and their use is complicated by risks such as amyloid-related imaging abnormalities (ARIA) and infusion-related reactions, along with high costs, which limit accessibility. Overall, these existing treatments have significant limitations, failing to halt disease progression and often struggling with the complexities of Alzheimer's pathology and the heterogeneous nature of patient symptoms. [11] However, donanemab emerges as a promising alternative, showing not only the potential to slow cognitive decline but also the ability to halt disease progression in some patients, particularly in the early stages of the disease. Its targeted mechanism and efficacy make it a notable advancement in the search for effective therapies for AD.
However, despite advances in studies, approaches to treating AD are scarce and mostly palliative, offering only moderate improvements. Currently used medications, including cholinesterase inhibitors and NMDA receptor antagonists, alleviate symptoms without treating or halting the disease. This limitation has compounded the search for disease-modifying strategies, especially those that address pathological changes in AD. To date, only two disease-modifying therapies have been approved for AD: lecanemab (Leqembi [Eisai R&D Management Co. Ltd., Nutley, NJ]) and aducanumab (Aduhelm [Biogen, Cambridge, MA]); however, the latter is no longer marketed. The requirement for further disease-modifying therapies is still significant because of the multifaceted and progressive nature of AD.
Donanemab is a monoclonal antibody that targets pyroglutamyl(3)-Aβ protein (3–42) to remove Aβ deposits and alter the disease course. One of its advantages is the opportunity to discontinue the treatment if the plaque is cleared. The U.S. Food and Drug Administration (FDA) has effectively approved the Kisunla (donanemab-azbt) injection for the treatment of AD in patients with mild cognitive impairment or mild dementia, in which the drugs were tested in clinical trials. Kisunla is given intravenously, and the recommended dose is given every 4 weeks [12, 13].
Thus, AD presents as one of the most complex neurological disorders with limited therapeutic interventions that can alter disease progression. Discoveries in the field of translational medicine offer novel therapies, with Donanemab serving as an example of successful targeting of Aβ plaques, which are considered one of the key pathophysiological features of AD [14]. This review aims to explore the history of donanemab from bench to bedside. Hence, through discussions on the mechanism of action, pharmacokinetics, and clinical use, we hope to gain a comprehensive understanding of the drug's use in the management of AD. Furthermore, we discuss the challenges and future prospects of donanemab to understand the real-world connections between basic and clinical research.
2 Donanemab's Mechanism of Action
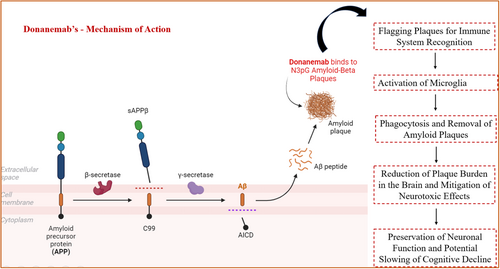
Donanemab is a monoclonal antibody that targets a modified form of Aβ called N3pG found in amyloid plaques of AD, which comprises the accumulation of misfolded proteins [15, 16]. Through this interaction, it attaches to this particular epitope on amyloid plaques, resulting in the removal of amyloid plaques from the brain. Such an interaction causes microglial activation and enables plaques to be engulfed and cleared from the vicinity of neurons, as shown in Figure 1. This is because a decrease in amyloid deposition is expected to slow the progressive nature of AD [12]. On the molecular level, plaque clearance may eliminate the cause of synaptic pathology and reduce neuroplasticity, which are key components of total cognitive impairment in the disease. At the genetic pathway level, the action of donanemab could also affect the downstream signal transduction of APP processing, shifting the amyloid cascade for tau hyperphosphorylation and neurofibrillary tangles, which are the other pathological hallmarks of AD. The removal of amyloid by this process also leads to a decrease in the amyloid concentration in the whole brain. As a result, donanemab lowers the amyloid plaque load and averts the impact of amyloid toxicity that occurs in subsequent steps. This encompasses diminishing the inflammation elicited by a buildup of plaque, protecting other neurons from harm, and perhaps even halting, or at least the deterioration of, mental faculties tied to AD. Furthermore, a decrease in amyloid plaques may potentially stabilize or enhance synaptic functionality, which is necessary for cognitive processes. Donanemab is designed for patients with early symptomatic AD, either with MCI or with mild dementia stage of AD [17].
3 Pharmacodynamics and Pharmacokinetics of Donanemab
Donanemab is a monoclonal antibody that specifically binds and eliminates pyroglutamate-modified Aβ plaques in AD. It helps to remove these plaques by binding to them, thereby enhancing the activity of microglia, thus reducing the levels of amyloid deposition in the brain, as observed in 2024 clinical trials [18]. This reduction is associated with decreased rates of cognitive and functional deterioration in patients. It could also be postulated that donanemab influences tau, a subfacet of AD. However, it is linked with ARIA, including ARIA-E (edema), which necessitates close attention. However, these risks are counterbalanced by the fact that donanemab maintained plaque reduction over time and might have more dosing flexibility, furthering the drug's therapeutic promise [19, 20].
Donanemab is a humanized monoclonal antibody that has predictable pharmacokinetics and reaches peak plasma concentrations within hours of intravenous infusion. It is mainly localized in the plasma and extracellular space and penetrates the brain across the blood–brain barrier to bind Aβ deposits. As it is proteolytically cleaved into peptides and amino acids, donanemab is mainly eliminated through the reticuloendothelial system, the half-life of which ranges from 20 to 30 days. This permits dosing intervals of every 4 weeks or more. The pharmacokinetics of the drug do not change with age, and no dose adjustment is required in elderly patients. The key characteristics of donanemab are discussed in Table 1. Furthermore, donanemab does not involve the cytochrome P450 enzymes for metabolism and therefore is expected to have low potential for drug–drug interactions, which are desirable for the long-term treatment of AD [21].
| Alternative names | Donanemab (LY3002813) |
|---|---|
| Class | Humanized IgG1 monoclonal antibody |
| Mechanism of action | Targets N-terminal pyroglutamate of amyloid-beta; promotes the clearance of amyloid plaques from the brain |
| Route of administration | Intravenous infusion. |
| Pharmacodynamics | Significant reduction in amyloid plaques observed in patients with early symptomatic Alzheimer's disease. |
| Pharmacokinetics | Linear pharmacokinetics; half-life ~28 days; steady-state concentration achieved after approximately 6 doses given every 4 weeks |
| Most frequent adverse events | Infusion-related reactions, amyloid-related imaging abnormalities (ARIA-E), headache, dizziness, and nausea |
| ATC codes | WHO ATC code: N06DX02 (Donanemab) |
| Tmax | Time to maximum plasma concentration: ~48 h post-dose. |
4 Preclinical and Clinical Trials
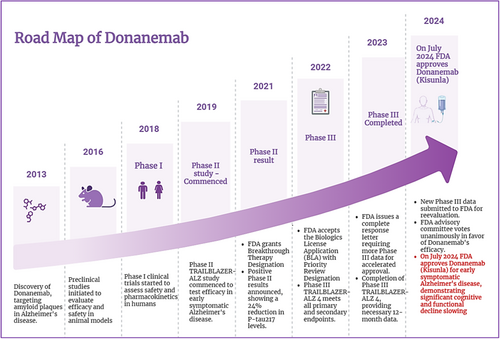
Preclinical and clinical trials of donanemab have demonstrated its efficacy in reducing amyloid plaques and slowing cognitive decline in Alzheimer's patients, leading to its recent approval, and summary of preclinical and clinical trials for donanemab is given in Table 2. The Road map shows the summary of the donanemab development timeline as shown in Figure 2.
| Trial phase | Objective | Participants | Dose | Results | References |
|---|---|---|---|---|---|
| Preclinical | Efficacy and safety in animal models | Transgenic mouse models | Various | Significant plaque reduction, cognitive improvements, no severe adverse effects | [22] |
| Phase I | Safety, tolerability, pharmacokinetics, initial efficacy | Small groups of healthy volunteers and mild AD patients | Various | Well-tolerated, common side effects included ARIA, preliminary amyloid reduction | [23] |
| Phase II | Efficacy and safety in early AD patients | 272 patients with early AD | 700 mg initially, then 1,400 mg | Significant amyloid reduction, slower cognitive decline, manageable ARIA incidences | [24, 25] |
| Phase III | Confirm efficacy and safety in a diverse population | 1736 patients with early symptomatic AD | 700 mg initially, then 1400 mg | Continued amyloid reduction, cognitive and functional improvements, nearly half with no progression at 1 year, 69% at 1.5 years | [26, 27] |
4.1 Preclinical Studies
Research in animal models has found that donanemab is effective in reducing amyloid deposition and associated neuropathological changes and has a relatively limited toxicity profile. It was found that the drug specifically and effectively decreased Aβ plaques in transgenic mouse models of AD and, as a result, there was a decrease in plaque density. In addition, donanemab enhanced the cognitive ability of these models through improved performance in memory and learning tests compared to untreated animals. Regarding the issue of safety, some findings concerning inflammatory aspects such as microhemorrhages or ARIA were mentioned; however, these effects proved to be fairly tolerable and dose-related. Donanemab exhibited a good tolerability profile and no significant toxicity on vital organs or organ systems at therapeutic concentration, providing the basis for progressing to phase I/II clinical trials [22].
4.2 Clinical Trials
4.2.1 Phase I Trials
Phase I trials aimed at assessing the safety profile, side effects, pharmacokinetics, and potential therapeutic effects of donanemab in humans [23]. These trials targeted a few selected healthy volunteers, as well as patients diagnosed with early-stage AD. Combinations of different doses were used to study tolerance levels. The study revealed that donanemab showed a tolerability profile at various doses. Other reported adverse events include infusion-related reactions and ARIA. It was also brought out from early study results that there was a decrease in Aβ plaques as well [28].
4.2.2 Phase II Trials
Phase II trials of TRAILBLAZER-ALZ have been designed to investigate the effectiveness and side effects of donanemab in a more extensive group of patients diagnosed with early AD. These randomized, double-blind, placebo-controlled studies, respectively, entailed about 272 patients. Donanemab was administered at a starting dose of 700 mg during the initial three doses and 1400 mg thereafter [12, 29]. Preliminary analysis revealed a significant decrease in the levels of Aβ plaques, the existence of which was established by PET. In this case, the patients had better cognitive outcomes when they were given donanemab than when they were given a placebo in terms of iADRS and Clinical Dementia Rating-Sum of Boxes (CDR-SB). The reaction observed in ARIA was more or less controlled with dosage modification and constant observation [24, 25].
4.2.3 Phase III Trials
Phase III trials TRAILBLAZER-ALZ 2 (NCT04437511) sought to establish the performance underway of donanemab within a broader and diversified population of patients [30]. These were large-scale, multicenter, randomized, double-blind, placebo-controlled clinical trials carried out using approximately 1736 patients suffering from early symptomatic AD. The dosing schedule was similar to that of phase II, with some modifications depending on the response of the patients. The outcomes showed sustained significant decreases in Aβ plaques as well as enhanced cognition and functioning. Nearly half of the participants showed no clinical progression at 1 year, and 69% achieved this milestone by 1.5 years. The overall benefit–risk profile was favorable, supporting donanemab's potential as a disease-modifying therapy for AD [26, 27].
5 Efficacy, Safety, and Tolerability
In clinical trials, the efficacy of donanemab was proven to be effective in treating AD [17]. The main outcomes in the trials were mainly the degree of Aβ plaque deposition and the rate of cognitive decline using recognized clinical rating scales such as the CDR-SB and the AD Assessment Scale-Cognitive Subscale (ADAS-Cog). Donanemab achieved these primary objectives by presenting impressive differences in lowering amyloid plaques together with a potential decline in the rate of cognitive decline compared with placebo. Secondary outcome measures included activities of daily living and also global cognition, which all had better improvements in favor of donanemab demonstrating the ability of the patients to perform daily activities and quality of life [31].
Despite the promising results of donanemab, clinical trials have reported several side effects. Thus, side effects include mild to moderate infusion-related reactions, such as headaches, fever, and chills, which may only require some dose manipulation or symptomatic treatment. The serious adverse effect profile included ARIA, where one of the serious side effects observed was ARIA-E (edema) and ARIA-H (hemorrhage). Side effects in the form of ARIA events were reported in a large number of patients, especially at higher doses, but were mostly controllable by close monitoring and dose adjustment [32]. Thus, concerning ARIA symptoms, most of the cases did not have any symptoms at all or had only mild signs, while some of the patients had more severe reactions that required some sort of intervention. Other less common but more serious side effects were identified to be hypersensitivity reactions and serious infusion reactions [21, 33].
The management of ARIA with donanemab entails serial magnetic resonance imaging (MRI) to assess the emergence of ARIA symptoms. Self-report questionnaires and periodic physician examinations were the most prevalent tools for evaluating symptom severity. There have been changes in the dosage of donanemab based on ARIA, and symptomatic treatment involving corticosteroids may also be administered. Further, health education, including the diagnosis of ARIA and the timely reporting of any complications, is offered to the patients and their caregivers [34, 35].
In general, the tolerability of donanemab has been favorable and well-evidenced in clinical trials. From the reports by patients and observations made, most participants pointed to the ability to continue taking the treatment without interference from their normal lives. Although side effects associated with ARIAs were apparent, many of them were asymptomatic or only mildly expressed, and treatment with the drug was well tolerated with further application of adherent management measures [36]. The rate of serious adverse events was not very high, and the outcome of donanemab in reducing amyloid plaques and slowing the cognitive decline outweighs the side effects. The drug is considered to have a favorable tolerability profile when compared to other drugs that target amyloid based on the fact that the side effects are usually manageable by monitoring for such effects and intervening appropriately [37, 38].
5.1 Comparison of Efficacy and Safety of Donanemab With That of Lecanemab and Aducanumab
The anti-Aβ antibodies donanemab, lecanemab, and aducanumab differ greatly in their efficacy and safety, and all are designed to diminish amyloid plaques in patients with AD. Targeting N3pG amyloid, donanemab has demonstrated a slower cognitive decline by 32% in the TRAILBLAZER-ALZ 2 study [24], with some patients showing an end to disease progression, particularly among those with light-to-moderate tau pathology. In opposition, lecanemab, which aims at amyloid protofibrils, moderates cognitive decline by 27% according to the CLARITY-AD trial, whereas aducanumab, targeting accumulated Aβ, has produced conflicting clinical outcomes with no consistent evidence of stopping disease progression [39]. The safety profiles vary; donanemab has a 27.3% ARIA risk primarily involving ARIA-E and ARIA-H, while lecanemab shows a 12.6% lower incidence of ARIA. Approximately 41% of patients suffer from the highest ARIA risk attributable to Aducanumab. Each therapy requires routine MRI monitoring for ARIA and is associated with infusion reactions. Both donanemab and Aducanumab are given through an intravenous infusion every 4 weeks, in contrast to lecanemab, which is delivered every 2 weeks [17, 33].
The novelty of donanemab lies in its potential to do more than just slow cognitive decline; it may actually stop the development of AD in some patients, which could make it a significantly more influential therapy for early Alzheimer's cases. Although the results are encouraging, careful patient selection along with persistent monitoring is necessary because of the associated ARIA risks. All these therapies differ in their combinations of effectiveness, safety, and frequency of administration, with comparable costs among the groups. Although donanemab holds the biggest potential for changing the trajectory of the disease, the continuing challenges concerning safety and monitoring are important factors in considering its clinical application [34].
6 Regulatory Status
6.1 Approval Status
In the United States, donanemab was approved by the FDA in April 2024 once post-marketing clinical trials that established the benefits and safety profile of donanemab were conducted. It is now also available for patients with early symptomatic Alzheimer's according to existing routine care disease control plans. Finally, the European Medicines Agency (EMA) recently gave full marketing authorization to donanemab in June 2024. Donanemab is available in EU member countries and has conditions and patient eligibility similar to those in the United States. The drug has been submitted or is being reviewed in other major markets; a Canada and Japan decision is expected by the end of 2024 to mid-2025 [40].
6.2 Regulatory Challenges
In 2023, donanemab faced a significant regulatory challenge when it was not approved by the EMA. The EMA's Committee for Medicinal Products for Human Use issued a negative opinion on donanemab's application for conditional marketing authorization [41]. In 2023–2024, donanemab met several important regulatory milestones to approve the drug in July 2024. A major issue in response to this was the implementation of strict monitoring procedures in the clinical trials, such as MRI scans at specified intervals to identify ARIA. To address the concerns raised by the safety authorities, the dosing regimen was modified in patients presenting with ARIA, and recommendations for patient selection and treatment management were provided to the practitioners. The purpose of these measures was to prevent adverse effects as much as possible but not at the expense of therapeutic outcomes. Perhaps the most significant difficulty was proving that donanemab had the ability to slow the progression of cognitive impairment, which meant that the drug had to meet strict clinical outcomes [42]. The TRAILBLAZER-ALZ 2 trial was a randomized, double-blind study that incorporated both cognitive and functional endpoints, including the use of Integrated Alzheimer's changes. Modifications were made to the primary and secondary endpoints, and the analysis of the trial outcomes revealed a statistically significant improvement in the prevention of cognitive decline. Donanemab was first approved with accelerated approval on the basis of intermediate endpoints of amyloid plaque burden, due to which it has now been approved based on long-term results for clinical efficacy [43].
Another regulatory area is inclusiveness in clinical trials, especially regarding the participation of different groups of people. Previous studies have criticized the underrepresentation of minority groups. The TRAILBLAZER trials included measures to enhance patient diversity through collaboration with community healthcare organizations and outreach programs. These efforts helped to confirm that donanemab was safe and effective in all population subgroups, which was important to address the regulatory concerns about the drug's scope. Finally, procedural filing was also a concern since the authorities wanted to be provided with factual evidence of how their profits outweighed the risks of taking donanemab. Lilly actively communicated with the relevant regulatory authorities throughout the trial and submitted data from the interim analysis for review and consideration. Finally, full approval was given to donanemab in 2024 after proving its safety through several phases of clinical trials and providing long-term data on safety and efficacy. These efforts highlighted the exhaustive process that donanemab went through to gain its status as a potential treatment for AD [42].
However, it is still important to note that there are certain obstacles encountered by donanemabs in terms of regulation. Some of the concerns have, however, been that further post-marketing surveillance is necessary to ascertain the drug's sustained effectiveness when taken over the long term. They are meant to confirm the long-term efficacy of the intervention and to reveal any side effects that may occur only occasionally and thus have not been observed in previous studies. In addition, there is still concern about the management of ARIA [44]. They persistently demand solid safety management systems to tackle these problems and prove that the advantages in cured cases of AD through donanemab exposure are greater than the impact for patients applying the medicine beyond clinical trials in practice. Overall, donanemab's regulatory journey reflects both the progress in AD treatment and the importance of comprehensive safety and efficacy evaluations in bringing new therapies to market [45].
7 Comparison With Existing Treatments
Compared with other Alzheimer's drugs, such as cholinesterase inhibitors and NMDA receptor antagonists, donanemab has unique features and efficacy profiles. Donepezil, Rivastigmine, and Galantamine are common cholinesterase inhibitors that act mainly by increasing the content of acetylcholine in the CNS, which improves cognitive function. However, they are only moderately effective, as they are basically used to treat the symptoms, and most of the time, the effects do not last for more than 6–12 months. These drugs were found to reduce dopaminergic cognitive decline only slightly with side effects such as nausea, vomiting, diarrhea, and fatigue and are relatively ineffective, particularly in the later stage of AD [46-48]. On the other hand, NMDA receptor antagonists such as memantine work by acting on glutamate, whereby they block it and provide symptomatic relief. Their benefits are similarly short-term; some data indicate that they may help delay cognitive decline in some patients, but the effect is likely to be moderated in patients with mild cognitive impairment. Data like dizziness, headache, confusion, and so forth also reduce their usage [49].
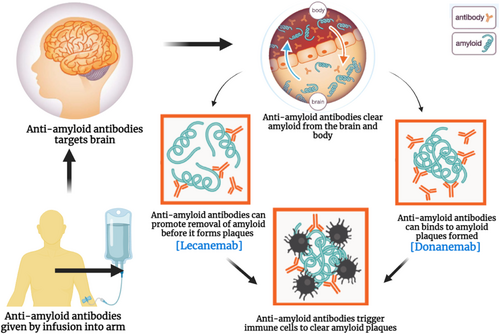
In contrast, studies have shown that donanemab selectively binds to N3pG amyloid plaques, slowing down cognitive deterioration by 32% and further arresting the disease in patients with early-stage AD. This disease-modifying ability makes donanemab superior to most existing treatments, which simply do not impact the disease's progression [21]. Though donanemab is associated with certain risks, including ARIA and infusion-related reactions, it is a step forward in treatment. Hence, the current use of cholinesterase inhibitors and NMDA receptor antagonists for treating forgetfulness primarily focuses on symptom alleviation, with no significant long-term effects on dementia reduction [37]. However, donanemab appears to be more effective in this context, as it initiates the process of altering the progression of AD and significantly slows down the worsening of dementia. Besides donanemab, other amyloid-modulating therapies such as lecanemab and aducanumab seek to alter the disease trajectory in patients with AD. Lecanemab, which targets amyloid protofibrils as illustrated in Figure 3 above, has been shown to slow down cognitive deterioration by 27% like other conventional drugs but cannot arrest the progression of the disease [39]. Aducanumab, while approved for its ability to reduce amyloid plaques, has produced mixed results in clinical efficacy, with some studies suggesting only variable cognitive improvement [10]. Both donanemab and Aducanumab have ARIA and infusion reactions, as does Lecanemab. However, given that donanemab acts differently and has different clinical outcomes, it may be a more effective medication to reduce further cognitive deterioration and stop the progression of the illness, which may be a significant advance in the treatment of AD. Table 3 shows a comparison of AD treatments: mechanisms, efficacy, and market status.
| Drug | Mechanism of action (MOA) | Approval status | Target population | Administration route | Safety and side effects | Comparative efficacy | Regulatory and market position | References |
|---|---|---|---|---|---|---|---|---|
| Donepezil | Acetylcholinesterase inhibitor, increases acetylcholine in the brain. | FDA-approved in 1996 | Mild to moderate Alzheimer's disease patients. | Oral tablets, orally disintegrating tablets, or liquid solution. | Nausea, diarrhea, insomnia, muscle cramps, and fatigue. | Symptomatic treatment only; no impact on disease progression or amyloid pathology. | Widely used for symptomatic relief; well-established in clinical practice. | [48, 50] |
| Rivastigmine | Inhibits both acetylcholinesterase and butyrylcholinesterase, increasing acetylcholine levels. | FDA-approved in 2000 | Mild to moderate Alzheimer's disease patients. | Oral capsules, liquid solution, or transdermal patch. | Nausea, vomiting, dizziness, diarrhea, and skin reactions (with patch). | Symptomatic treatment only; no impact on disease progression or amyloid pathology. | Established symptomatic treatment but facing challenges with market penetration and use. | [46] |
| Galantamine | Acetylcholinesterase inhibitor, also enhances cholinergic function through interaction with nicotinic receptors. | FDA-approved in 2001 | Mild to moderate Alzheimer's disease patients. | Oral tablets, extended-release capsules. | Nausea, vomiting, diarrhea, and dizziness. | Symptomatic treatment only; no impact on disease progression or amyloid pathology. | Established symptomatic treatment, with recent formulations improving patient adherence. | [47] |
| Memantine | NMDA receptor antagonist, regulates glutamate activity. | FDA-approved in 2003 | Moderate to severe Alzheimer's disease patients. | Oral tablets or liquid solution. | Dizziness, headache, constipation, confusion. | Symptomatic treatment; may provide benefits in advanced stages. | Established for moderate to severe stages, widely used. | [49, 51] |
| Donanemab | Targets amyloid plaques by binding to a pyroglutamate-modified form of Aβ, helping clear plaques. | FDA-approved in July 2024 | Early symptomatic Alzheimer's disease patients. | Intravenous (IV) infusion, typically every 4 weeks. | ARIA, infusion-related reactions, increased risk of amyloid-related imaging abnormalities (ARIA-E and ARIA-H). | Potentially more effective than older amyloid-targeting therapies and similar in efficacy to Lecanemab, with a focus on specific Aβ forms. | FDA-approved with growing clinical use; considered promising for early Alzheimer's. | [24, 52] |
| Aducanumab | Monoclonal antibody targeting aggregated amyloid-beta (Aβ) to reduce amyloid plaques. | FDA-approved in June 2021 (limited market uptake) | Mild Alzheimer's disease patients with confirmed amyloid pathology. | Intravenous (IV) infusion every 4 weeks. | ARIA (including ARIA-E and ARIA-H), headache, microhemorrhages, and edema. | First in class; overshadowed by newer treatments like Lecanemab and Donanemab in terms of cognitive benefits. | Limited market uptake due to efficacy controversies; future uncertain. | [53, 54] |
| Lecanemab | Monoclonal antibody targeting amyloid-beta protofibrils to reduce amyloid plaque formation. | FDA-approved in January 2023 | Mild cognitive impairment or mild Alzheimer's disease with confirmed amyloid pathology. | Intravenous (IV) infusion every 2 weeks. | ARIA, headache, infusion-related reactions, microhemorrhages, and bleeding events. | Demonstrated strong efficacy in slowing cognitive decline in Phase III trials, possibly more effective than Aducanumab. | Gaining popularity due to positive long-term data; seen as promising for early Alzheimer's. | [39, 55] |
8 Challenges and Future Prospectives
Donanemab, a novel therapy for AD, has several advantages and risks. Although they demonstrated promising results in several clinical trials, the long-term safety and effectiveness of donanemab are still unknown because the data obtained are only based on a limited number of months starting from its approval in July 2024. The main effects of a treatment are rather different from one patient to another, depending on genetically mediated factors like APOEε4 and the type of AD; thus, it is challenging to predict who could benefit from the treatment the most. Moreover, the administration of donanemab through intravenous infusions poses significant challenges in terms of cost and method, particularly in settings with limited resources, raising concerns regarding health disparities. Other clinical concerns associated with the manifestation of lesions, such as ARIA-E and ARIA-H, might require dose adjustment or discontinuation of therapy due to the risk in elderly or more vulnerable patients. In addition, donanemab is under heavy competition from other newly approved anti-amyloid agents such as lecanemab and other existing symptomatic controls, which may reduce its market acceptance and contribute to the shift of AD therapeutic modalities. Regulatory issues are also, however, not fully resolved, since post-marketing surveillance may once again discover other safety issues or may result in a limitation of drug use. The strict biomarker-based patient inclusion, which requires access to imaging techniques such as amyloid PET and tau imaging, may impose restrictions on drug availability, thus curbing its role in the clinic.
There are bright perspectives in the future development of donanemab targeting various diseases, and among them is the perspective of its use in the concept of personalized medicine where the drug can be used in combination with other drugs according to the biomarkers and genetic characteristics of patients. Applying donanemab in synergy with other peripheral amyloid-clearing techniques that are in planning with targeted tau inhibitors or gene therapies may unveil the efficacy of the drug. Further research should focus on the effects of combination therapies using donanemab to address several pathological properties of AD to facilitate better treatment interventions. Further, we may also be able to explore new applications for donanemab beyond early symptomatic AD, including preclinical states, or in circumstances involving Alzheimer's with mixed features or vascular components. Potential enhancements in the method of drug administration, such as subcutaneous preparations or slow-release depot systems, may also help increase the use of donanemab given the inconvenience of infusions. With the enhancement of diagnostic tools, especially in biomarkers in the bloodstream and advanced imaging, the identification of potential patients for donanemab treatment may also become more effective and noninvasive, thus increasing the usage rate. Moreover, future studies, as well as the amass of longitudinal and real-world data, may even afford ways of fine-tuning the application of donanemab in actual clinical practice with risks reduced to a minimum while the benefits are simultaneously reaped to the fullest.
9 Conclusion
Donanemab has already been recognized as a game changer in the management of AD and is a clear example of successful translational medicine. Its development is a step forward in efforts to create a bridge from basic research to clinical application, providing new hope to Alzheimer's patients. In prior experiments conducted on animal subjects, treatment was found to be useful in eliminating amyloid plaques and moderate side effects. Furthermore, Phase I to III trials supported that donanemab had good potential in reducing cognitive decline and amyloid plaque accumulation. The risk factors of ARIA events and infusion reactions were predictable, and the drug was well tolerated. There has been enormous regulatory advancement because donanemab has gained full approval from both the FDA in April 2024 and the EMA in June 2024 due to demonstrable clinical value and safety. Compared with the currently available drugs, such as donepezil and rivastigmine, donanemab has been shown to exhibit better plaque regression and cognitive enhancement than other amyloid-targeted drugs, but with similar side effects. Future studies will focus on the enhancement of administration protocols, extension of the scope of application, and use of multiple agents. Any eventual loss of efficacy or adverse effects will likely be unveiled in follow-up investigations of donanemab, an essential piece in the Alzheimer's treatment puzzle. The ability to change the disease course, possibly in earlier stages, and perhaps in other neurodegenerative diseases, provides hope for better results. The combination of donanemab with superior biomarkers and imaging modes with further enhancements of gene and protein-centered editing places a new wave of precise prescriptions in Alzheimer's therapy. For these reasons, it is crucial to maintain the research focus and develop further to better comprehend AD and improve patients' outcomes, focusing on donanemab as a path to further neurodegenerative disease treatment.
Author Contributions
Nandhini Jayaprakash: writing–review and editing, writing–original draft, visualization, validation. Karthikeyan Elumalai: investigation, formal analysis, conceptualization.
Acknowledgments
The authors have nothing to report.
Ethics Statement
Ethical approval was not required as this is a review of previously published studies.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.




