Protocol for the development and validation of a clinical measurement tool for fear of disease progression and recurrence in cardiac patients
Abstract
Introduction
One in two cardiac patients fear having another heart event or their heart condition getting worse. Research in other chronic illnesses demonstrates that screening for fear of progression and recurrence is vital for adequately addressing such concerns in clinical care. The current project aims to develop and validate a measure for fear of progression and recurrence in cardiac patients.
Methods
The Fear of Cardiac Recurrence and Progression Scale (FCRP) will be developed through a multistep process. An initial item pool will be generated through a review of the literature and existing measures and consultation with and feedback from key informants. The item pool will be tested in a sample of over 250 adults who have ever had an acute coronary event, undergone cardiac surgery, or a chronic cardiac condition. Exploratory factor analysis will be used to identify the underlying factors, and Rasch analysis will be used to reduce the number of items. A short form version of the FCRP will be developed for use as a brief screening tool, informed by clinical relevance and Rasch psychometric indices.
Discussion
While many cardiac patients experience fears related to the progression or recurrence of their illness, there remains the need for a validated tool with which these concerns can be identified and measured. It is expected that the design and validation of the FCRP will aid identification of cardiac patients suffering from clinically significant levels of fear of progression and recurrence and facilitate the design of tailored psychological interventions to target these fears.
Highlights
-
The current project aims to develop and validate the Fear of Cardiac Recurrence and Progression Scale (FCRP).
-
The design of the FCRP will be guided by best practice in measurement development and validation and informed by patient experience and academic and clinical expertise.
-
Using the FCRP, health professionals will be able to identify patients in need of support for fears of cardiac disease progression, recurrence, and related consequences, facilitating referral to counselling services.
-
The creation and validation of the FCRP will facilitate the design of tailored psychological interventions to target these fears and support patient recovery.
1 INTRODUCTION
Fear of progression (FoP) is the fear that a disease will progress with all its consequences.1 Another common term is fear of recurrence (FoR), which is typically used when referring to a disease that remit-recurs rather than one that continually progresses.2 Such concepts were introduced to address the high level of distress in patients with chronic illnesses that could not be explained from a psychiatric perspective alone, as such fears were often deemed to be not out of proportion or irrational.1 While anxiety is inherently excessive or out of proportion to the actual danger posed,3 FoP is often an appropriate response to an extraordinary life event.1
FoP has been extensively characterized in oncology, specifically referred to as fear of cancer recurrence (FCR).4 FCR has been well conceptualized, with hundreds of research outputs resulting in a range of measurement tools to assess FCR (for review, see Simard et al.5) and development of specific FCR interventions (for review, see Pradhan et al.6). FCR is one of the most reported concerns for cancer patients, and a treatment need which often remains unmet.5 FCR is reported to affect around 30%–50% of cancer patients and survivors.7 For a substantial portion of patients, this fear is persistent and may worsen over time.5, 8
FoP is not well-characterized in illnesses other than cancer. A systematic review identified only 25 qualitative studies and 11 quantitative studies investigating FoP in diseases other than cancer.9 This is compared to the 130 quantitative studies identified by Simard et al.5 and the 87 qualitative studies identified by Almeida et al.10 in cancer patients, with the research outputs growing exponentially since this time. Nevertheless, the results of these systematic reviews support the contention that FoP is indeed transdiagnostic, with patients across multiple chronic illnesses reporting fears relating to progression, recurrence, dying, and becoming a burden to the family.9 In these patients, FoP was associated with poor quality of life and high depressive and anxiety symptoms.9
There has been increasing recognition of the need to consider FoP in cardiac patients.11-14 Qualitative studies in cardiac patients have repeatedly identified fears related to having another heart event, deteriorating heart and general health, dying, and related consequences.15-20 Furthermore, recent evidence suggests that such fears are widespread, with one in two cardiac patients reporting concerns about either having another heart event or their condition getting worse.21 Indeed, the few studies that directly investigate FoP in cardiac illness provide preliminary evidence that FoP is present in a large portion of cardiac patients and that this has important implications for both disease prognosis and general well-being.22-27
Nonetheless, research directly investigating cardiac FoP is scarce and limited due to the need for a validated tool with which to measure FoP in cardiac patients. In the systematic review of FoP in chronic diseases other than cancer,9 only three qualitative studies and one quantitative study were identified in cardiac patient populations. While more quantitative studies have been published since this review, all existing studies22-27 have used variations of a FoP measure designed for and validated in cancer, diabetes, and rheumatic disease patients.1, 28, 29 These measures have not been validated for use in cardiac patients. The nature of FoP, and therefore the content of a valid FoP measure, will depend on the characteristics of the disease, such as illness course, symptom presentation, and so on. For example, a measure for FoR in breast cancer patients includes a factor relating to “womanhood worries,”30 and a measure for fear of complications in diabetic patients includes items relating to developing kidney problems or losing one's eyesight.31 Consequentially, a measure designed for use in other illnesses will include issues and experiences irrelevant to cardiac patients and omit those that may be uniquely relevant to this population.
The development of a cardiac-specific measure is critical in addressing FoP in cardiac care. FoP is not a unitary fear; rather, this construct represents a different set of fears for each patient.10 A measurement tool should allow identification of the specific concerns patients may have to guide therapeutic intervention. Furthermore, a validated measure would allow clinicians in primary care settings to screen for psychosocial concerns that may otherwise not have been identified.32 Research in oncology demonstrates that patients may be reluctant to instigate a discussion around FoP to avoid appearing ungrateful or damaging their relationship with their clinician.33 Meanwhile, health professionals who are not specialists in mental health support may likewise experience discomfort discussing and managing FoP concerns.34 Importantly, FoP is not related to conventional prognostic factors such as disease severity and duration;26, 35, 36 thus, a patient's illness presentation is not a reliable indicator of the presence and severity of FoP. The provision of a clinical measurement tool may overcome these barriers by providing an avenue to address FoP without relying on patients or health professionals to raise this concern spontaneously. In addition, screening tools allow identification of the patients at greatest risk,37 facilitating referrals to specialist mental health services for patients in need.
However, no tool has been designed and/or validated to measure the specific concerns associated with disease progression and recurrence in cardiac patients. Following the lead of oncology, the creation and validation of such a measurement tool will facilitate the design of effective treatments to intervene with these fears in cardiac patients.
2 AIM
The aim of this study was to develop and validate the Fear of Cardiac Recurrence and Progression Scale (FCRP). Using the FCRP, health professionals will be able to identify fears relating to disease progression, recurrence, and related consequences in cardiac patients, thereby facilitating referral to and delivery of targeted psychotherapeutic interventions to support cardiac patient recovery, modelled of similar interventions trialled in cancer patients.6 Psychometric testing of the FCRP will result in the development of a short form to be used in settings such as outpatient clinics and cardiac rehabilitation programs to screen patients for FoP or FoR and facilitate appropriate referral to counselling services.
3 METHODS
3.1 Participants
3.1.1 Inclusion and exclusion criteria
Eligible patients will be adults, over the age of 18, who have ever had an acute coronary event such as acute coronary syndrome (ACS), acute myocardial infarction, undergone cardiac surgery, such as coronary artery bypass graft surgery, or who have a chronic cardiac condition such as heart failure and congenital heart conditions.
Patients who do not have adequate English language proficiency to read and understand the Participant Information and Consent Form (PICF) and questionnaire will be excluded. Participants who are completing the questionnaire online will need access to a computer, tablet, or smartphone, and the internet. Participants who do not have access to such means will be provided with the option to complete a hardcopy questionnaire.
The study has broad inclusion criteria to capture common fears relating to disease progression among a range of patients with cardiac conditions. It was decided that the study take this broad approach, rather than focussing on a specific cardiac illness or set of symptoms, for two reasons. First, many patients have multiple co-occurring cardiac illnesses, with the potential of having both acute and chronic symptoms, resulting in a large amount of heterogeneity between different patients, irrespective of primary diagnosis. Second, for this measure to be feasible to use within the intended clinical settings, it will need to be valid and applicable across a wide range of presentations and demographics.
3.1.2 Participant recruitment
Participant recruitment will occur through self-referral into the study. Participants will be informed about the study either in person by being provided a hardcopy flyer containing information regarding the study or online through a social media post or other communication. The flyer contains information about what is involved in the study, who is eligible to participate, how to register, and study contact information. This information will be available on the website for participants who are directed from an online source.
The study will be promoted online through website presence and social media campaigns by the affiliated research and endorsement bodies. The Australian Centre for Heart Health (ACHH) will also recruit through their Cardiac Counselling Clinic and a database of previous research participants who wish to have continuing research participation. The study will be communicated to cardiac health professionals at external sites, who will be asked to provide the study flyer to patients they believe are eligible or by placing the flyer in patient areas.
This research study has been endorsed by the Australian Cardiovascular Health and Rehabilitation Association (ACRA) and will also be promoted in ACRA networks and communications.
3.2 Design of the FCRP
The method described in this protocol for the development and validation of the FCRP was informed by the best practice for performing such a task, as outlined by Boateng et al.38
The item pool was finalized through two processes: (1) generation of items through reviewing both the literature and existing measures and (2) consultation with and feedback from key informants.
3.2.1 Literature review
The purpose of the literature review was to characterize the construct of interest; FoP, within the population of interest; cardiac patients; and to ensure no measure already existed that served this purpose. Identifying and defining the key construct of FoP or FoR involved reviewing how this has been conceptualized in oncology and other chronic illness research. This process also involved investigating to what extent FoP has been conceptualized in cardiac patients and confirming that no existing instrument specifically and validly measured FoP and FoR in cardiac patients.
To develop the initial item pool, existing measures were reviewed for items relevant to FoP or FoR in cardiac patients. This involved adapting relevant items from two sources: (1) existing FoP or FoR scales from other areas of chronic illness research and (2) existing measures of anxiety, fear, and distress in cardiac patients. Finally, cardiac patient concerns highlighted in previous qualitative and quantitative research aligned with the construct of FoP or FoR were adapted into item form. Items were discussed between the multidisciplinary investigator group throughout this process to obtain consensus on the relevance of items. Forty items resulted from this initial item generation process.
3.2.2 Key informant process
The second step of item generation involved collaboration with key informants, which served both as a method of item generation and as a content validity exercise. This was a three-stage process with different key informants at each stage. The first stage included 17 health professionals recruited for their previous experience working with cardiac patients or in areas with similar concerns, such as oncology. This group included cardiac rehabilitation coordinators and nurses, nurses in cardiac wards, exercise physiologists, mental health social workers, psychologists (both general and cardiac/health specific), and psychiatrists. Informants reported an average of 19 years working as a health professional and an average of 14 years working with cardiac patients. The second key informant group comprised five academic experts who had been involved in the recent design of a scale to measure distress in cardiac patients.39 Key informants in this group provided experience of both the concerns raised by cardiac patients and experience in designing and validating health measures. The final group comprised seven cardiac patients (five females, two males), aged between 60 and 87 years (average 71 years). Three patients within this group had experienced a heart attack and a percutaneous coronary intervention/stent, three patients had CAGBS, and one patient had atrial fibrillation.
At each stage, the key informant groups were provided with background information to understand the construct of interest and were asked to draw on their experience to provide an opinion on the structure and content of the scale. The investigator group made iterative changes to the item pool at each stage as documented in Table 1. At the conclusion of the key informant process, 44 items were included in the item pool.
| Group | Outcome |
|---|---|
| Health professionals | 7/17 key informants provided suggestions for change. This resulted in: |
|
|
| Academic experts | 5/5 key informants provided suggestions for change. This resulted in: |
|
|
| Cardiac patients | 5/7 key informants provided suggestions for change. No changes were made to the scale at this stage, as suggestions were already incorporated or were outside the scope of the current study. |
3.2.3 Item pool for testing
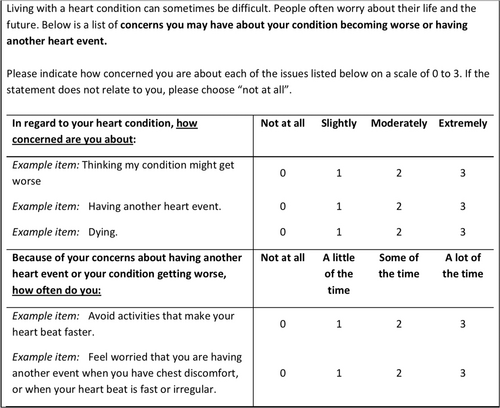
Items generated through the process outlined above were reworded where appropriate to ensure relevance to the measurement of cardiac FoP or FoR and appropriateness of fit with the following instruction and response set. The wording of the items, stem, and responses were developed from a combination of reviewing existing measures, key informant, and multidisciplinary group decisions. Items were separated into two groups which required different responses/stems, depending on whether they were addressing a fear or a behavioral response to fear. All responses were on a four-point Likert scale. This is illustrated in the example scale below (Figure 1).
3.3 Additional measures
In addition to the FCRP item pool, several other measures will be administered for validation purposes.
3.3.1 Demographic questionnaire
Basic sociodemographic, medical, and cardiac condition-related information will be collected using standard self-report questions used in previous ACHH-administered studies.39
3.3.2 Visual analog scale for FoP
Participants will be asked to rate their fear of their cardiac condition progressing or having recurrent events on a scale of 0–10, using either a slider (online completion) or thermometer (hardcopy completion) as a visual analog. This scale was designed from the one-item question FCR screener.40 The use of a single-item FoP measure has demonstrated good validity and reliability in cancer patients.40, 41
3.3.3 FoP Questionnaire Short Form (FoP-Q-SF)
FoP-Q-SF28 is a 12-item measure of FoP, adapted from the full 43-item measure.1 The FoP-Q-SF has demonstrated validity and reliability in breast cancer cohorts.28 Psychometric properties have not been assessed in cardiac patients.
3.3.4 Cardiac Distress Inventory Short Form (CDI-SF)
CDI-SF42 is a 12-item measure designed as a screener for distress in cardiac patients. The CDI-SF has a clinical cutoff score of ≥13 to determine patients who have clinically significant levels of distress. The CDI-SF has been established to have excellent internal consistency and good convergent and discriminant validity.42
3.3.5 Cardiac Anxiety Questionnaire (CAQ)
CAQ43 is an 18-item inventory designed to measure heart-focused anxiety in patients with and without heart disease. The CAQ has been validated in patients hospitalized with ACS, demonstrating good internal consistency and reliability.44
3.3.6 PTSD Checklist for DSM-5 (PCL-5)
PLC-545 is a 20-item measure assessing the 20 symptoms of posttraumatic stress disorder (PTSD) in the DSM-5 (Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition). This was designed to screen individuals for PTSD and formulate provisional diagnoses.
3.3.7 Mishel Uncertainty in Illness Scale-Community Form (MUIS-C)
MUIS-C46 is a 23-item scale designed to assess uncertainty in illness in nonhospitalized chronically ill people. This scale is adapted from the original MUIS with the removal of the items referring to treatment and communication with health professionals in an acute hospital setting. The MUIS-C has previously been used with a range of acute and chronic cardiac patients and has demonstrated moderate to good internal consistency within this population.47-50
3.3.8 Patient Health Questionnaire-9 (PHQ-9)
PHQ-951 is a brief nine-item depression tool based on the symptoms of major depressive disorder. The PHQ-9 has been endorsed by the National Heart Foundation of Australia as the recommended tool for depression screening in cardiac patients.52
3.3.9 Generalized Anxiety Disorder Instrument (GAD-7)
GAD-753 is a seven-item self-report measure to identify generalized anxiety in primary care. The GAD-7 has good reliability and validity for detecting generalized anxiety.54 The GAD-7 has been validated for use in cardiac populations.55
3.3.10 Metacognitions Questionnaire-30 (MCQ-30)
MCQ-3056 assesses individual differences in metacognitive beliefs, specifically addressing the five factors important to the metacognitive model of psychological disorders. The MCQ-30 has demonstrated good validity and reliability.56 An 18-item subset of the MCQ-SF will be used, including only the factors on positive beliefs, negative beliefs, and need for control, due to the higher association between these subscales and FoR.57, 58 This approach has previously been taken in a study of fear of disease progression and recurrence in cancer.59 The use of the individual factors rather than the full five-factor measure is supported by validation studies of the MCQ-30 in a sample of distressed cardiac patients, which demonstrated that while each factor demonstrated good internal consistency, and may individually explain anxiety and depression symptoms among cardiac patients, the full five-factor model did not demonstrate good fit in this population.60
3.3.11 Short Form Health Survey (SF-12)
SF-1261 is a 12-item measure of health-related quality of life. The SF-12 has good reliability and validity as a generic measure of health-related quality of life in cardiac populations62, 63
3.4 Procedure
Consenting participants will complete the PICF and questionnaire pack, including the FCRP and additional measures listed above. Participants completing the study online will gain access to the PICF and questionnaire pack through a secure online server hosted through the Research Electronic Data Capture (REDCap) platform. Participants can also telephone ACHH to request a hardcopy questionnaire if they do not have access to the internet or prefer not to complete the questionnaire online. These participants will be posted the PICF and questionnaire pack with a reply-paid envelope. No identifying information (name, address, and date of birth) will be collected as no patient follow-up is required. The questionnaire will take approximately 20 min to complete. This time was estimated by completion of the survey by the study team and confirmed by assessing response time of the first 10 participants (average of 23 min).
3.5 Data analysis
3.5.1 Development of the FCRP
Statistical analysis will be modelled from the methodology utilized in the design and validation of the CDI.39
Establishing the dimensions of the FCRP
Exploratory factor analysis (EFA) will be used to identify any latent constructs measured by the FCRP and establish the factors of the FCRP. The optimal number of factors will be informed by parallel analysis of all items.64, 65 The suitability of data for EFA will be assessed using the Kaiser–Meyer–Olkin measure of sampling adequacy, Bartlett test of sphericity, and inspection of the scree plot. Principal axis factoring will be used to estimate the factor structure. A number of nonorthogonal rotation techniques such as oblimin, promax, and simplimax will be tested to assess the best model fit in consideration of clinical and theoretical interpretation of factor loadings. Variable loadings lower than 0.32 will be suppressed to assist interpretation of the EFA solution.66 Model fit will be further assessed using multiple tests, including χ2, the standardized root mean square residual, root mean square error of approximation, Benteler's comparative fit index, and Tucker–Lewis index.67, 68
Item reduction
Rasch analysis will be used to reduce the number of items included in the FCRP. Rasch analysis is a technique used to assess how well a set of items represents an underlying latent construct. Rasch analysis will be used to assess the fit of items within each factor identified in the EFA. Rasch analysis will be conducted using the WINSTEPS software, version 5.2.2. (https://www.winsteps.com). The rating scale model will be used to assess item fit in an iterative process whereby estimates are repeated, with model fit being checked and item deletion occurring at each iteration, until the necessary criteria for the Rasch parameters are met. Rasch analysis assumes unidimensionality, that is, all items within the tested factor belong to one latent construct. This will be assessed using point measure correlations, principal components analysis of the Rasch residuals, and Wright's unidimensionality index. The assumption of local independence, that is, each item functions independently of responses to another item, will be assessed by inspection of residual correlations, and the assumption of monotonicity will be evaluated through the assessment of the rating scale of each item. The fit of individual items to the factor will be assessed using infit (information-weighted) and outfit (outlier sensitive) mean square statistics and standardized z-scores. Item and person separation indices will be calculated to assess the spread of items and individuals along a continuum of endorsement of each factor. Item and person reliability indices will be calculated to assess the reliability of rater observations and consistency of items within a factor. Differential item functioning (DIF) will be assessed to analyze the extent that items function differently across different subgroups for age, sex, and education. A criterion of at least 0.5 logit difference with a p < 0.05 (according to the Rasch–Welch test) will be used to detect problematic DIF.69
3.5.2 Psychometric properties of the FCRP
Evaluating the psychometric properties of the FCRP will be informed by the Consensus-based Standards for the Selection of Health Measurement Instruments (COSMIN).70, 71
Reliability
Internal consistency of the FCRP will be determined using McDonald's omega72 and evaluation of the reliability indices from the Rasch analysis.
Validity
Concurrent validity will be established by assessing the associations between FCRP scores and FoP-Q-SF and the visual analog scale. It is not possible to use another measure specifically for cardiac FoP or FoR, as none exists. Discriminant validity will be measured through the association between FCRP scores and established scales of measures of other constructs (such as the PHQ-9, GAD-7, PLC-5, and MUIS-C).
3.5.3 Development of a short form
It is intended that the short form version of the instrument (FCRP-SF) will be used in clinical settings to screen for FoP or FoR in cardiac patients. Use of the FCPR-SF will determine whether administration of the full FCRP is indicated and can aid in referral to psychocardiology services. The development of the FCFP-SF will be informed by clinical relevance and Rasch psychometric indices. Several options for short forms will be analyzed for suitability: a short form including items from each factor, a single factor short form, and the use of the visual analog scale as a screening measure. Rasch fit indices will be used to evaluate the suitability of items for the short form. First, items with infit and outfit mean squares outside the 0.6–1.4 range and standardized fit statistics outside the ±2.0 range will be identified as possible candidates for deletion.73 Items will be excluded one at a time, recalculating fit statistics for the remaining items after each deletion, while monitoring the resulting overall item level fit. Person reliability will be examined after each deletion to ensure that the remaining item set is well distributed across different levels of the latent measurement scale as measured by the Wright Map. All Rasch fit parameters, including person reliability and separation, will be calculated for the resulting FCRP-SF and compared to the original FCRP. Further, concurrent and discriminant validity will be assessed as explained above for the FCRP. Receiver-operating characteristics will be used to identify the optimal cutoff score for the FCRP-SF in which it causes clinically significant distress, using the establishing cutoff score for the CDI-SF. Final decisions regarding whether to retain or delete an item will be based on the clinical importance of the content. Thus, the design of the FCRP-SF will include clinical oversight from a multidisciplinary team of experts within the field.
3.5.4 Sample size requirements
Recommendations of sample size for EFA in instrument development are that there should be at least five cases for each item in the instrument being used.74 Rasch modelling for exploratory purposes should be based on at least N = 100 and preferably N = 250.75
3.6 Timeline
The study will take 2 years, with a timeline as follows: Months 1–6: Development of the FCRP item pool and study setup. Months 7–18: Trialling the FCRP with cardiac patients. Months 19–21: Completion of data analysis resulting in the development of the FCRP and FCRP-SF. Months 21–24: Dissemination of study findings.
3.7 Progress to date
At the time of writing this paper (September 2023), the study has been underway for 10 months. The item pool has been developed, as outlined previously, and patient recruitment commenced in May 2023. As of September 1, 2023, 90 cardiac patients have accessed the item pool and completed the questionnaire pack. Monthly recruitment targets have been met to date. It is anticipated that the results of this study will be published in the second half of 2024.
4 DISCUSSION
While many cardiac patients experience fears related to the progression or recurrence of their illness, there is no validated tool with which these concerns can be measured. The current paper outlines a protocol for a project to develop the FCRP, together with the FCRP-SF. The design and validation of the FCRP will be guided by best practice in measurement development and validation38, 70, 71 and informed by patient experience and academic and clinical expertise. The design and validation of the FCRP will facilitate future research, where there are currently limited methods through which to quantify FoP in cardiac patients. Using this measure, future research can better characterize the prevalence and impact of cardiac FoP and identify the patient groups who may be at most risk. In practice, the FCRP will provide health professionals with a tool to assess the specific fears and concerns experienced by their cardiac patients so that these may be addressed. The development of the FCRP-SF will further aid the identification of patients most in need of support, in a range of primary, secondary, and tertiary settings in which health professionals may be time-poor and facilitate referral to psychological services. It is anticipated that the successful completion of the current project will facilitate the design of tailored and effective interventions for FoP in cardiac patients.
AUTHOR CONTRIBUTIONS
All authors were involved in the conceptualization and development of this project. Sarah T. Clarke drafted the article, and all authors contributed to and have given final approval for the current version to be published.
ACKNOWLEDGMENTS
This research is supported through an Australian Government Research Training Program Scholarship.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
This study has been approved by the University of Melbourne Humans Research Ethics Committee (2023-25413-39497-5) to run from April 20, 2023 to April 20, 2026.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.




