Primary Squamous Cell Carcinoma of the Gallbladder: Case Report
Funding: The authors received no specific funding for this work.
ABSTRACT
Pure gallbladder squamous cell carcinoma is rare. Adenocarcinoma is the foremost malignant pathology of gallbladder cancer. Simultaneously, squamous cell carcinoma accounts for only 1% of malignant gallbladder tumors. They are often advanced at diagnosis and associated with a poor prognosis. The patient was a 37-year-old man diagnosed with poorly differentiated squamous cell carcinoma of the gallbladder. Cholecystectomy, right hemicolectomy, hepatic wedge resection, and regional lymph node dissection were performed, and then he received six courses of adjuvant chemotherapy with the FOLFOX regimen. After 2 months, liver metastasis was diagnosed, and a chemotherapy regimen consisting of gemcitabine, cisplatin, and pembrolizumab was started. After three courses, stable disease was seen. Surgery is the main treatment. There is no consensus about the best adjuvant treatment. The results obtained from this patient might help elucidate the role of adjuvant therapies in treating this rare case report.
Summary
- Gallbladder squamous cell carcinoma is a rare disease.
- Surgery is the main treatment.
- There is no consensus on the best adjuvant treatment.
- The results obtained from this patient might help elucidate the role of adjuvant therapies in treating this rare case report.
1 Introduction
Pure gallbladder squamous cell carcinoma is rare [1]. Adenocarcinoma is the foremost malignant pathology of gallbladder cancer. Simultaneously, squamous cell carcinoma accounts for only 1% of malignant gallbladder tumors [2]. Patients with squamous cell carcinoma are associated with higher grades and poorer prognosis [3]. According to the analysis of the national cancer database, during 11 years, 1084 cases of gallbladder squamous cell carcinoma were identified [4]. Abdominal pain is the most common symptom [5]. They are often advanced at diagnosis and associated with a poor prognosis [1]. In this study, a case of pure squamous cell carcinoma is reported, which has been treated with radical surgery and adjuvant chemotherapy.
2 Case History and Examination
A 37-year-old man was admitted to hospital because of a 6-month history of chronic right upper quadrant abdominal pain. The pain was progressively getting worse over the months. He was vitally stable on physical examination, and moderate tenderness in the right upper quadrant was present. His Karnofsky performance status was 70–80 (normal activity with effort with symptoms of disease). There was no specific disease in the family or personal history.
2.1 Differential Diagnosis, Investigations, and Treatment
Laboratory studies showed mild leukocytosis (WBC count of 15,600/μL with 74% neutrophils, Hb: 12 g/dL, Plt: 250 × 103 cells/mm3), liver function tests (AST: 17 U/L, ALT: 18 U/L, ALP: 105 IU/L, total bilirubin: 1.08 mg/dL) and kidney function tests (urea: 32 mg/dL, creatinine: 0.7 mg/dL) were reported as normal. A CA19-9 level of 45 U/mL (normal: < 37 U/mL) was reported, and CEA was not checked.
The patient was evaluated by transabdominal sonography, which showed a heterogeneous hypoechoic mass that arises from the gallbladder fundus measuring 79 × 67 mm with invasion to hepatic parenchyma. A computed tomography (CT) scan of the abdomen with and without contrast injection was done. A large enhancing solid mass with a necrotic center in the left subhepatic region in favor of gallbladder origin with fat stranding and regional lymphadenopathy with short axis diameter (SAD) 6 mm and invasion to segment V of the liver and transverse colon was observed (Figure 1).
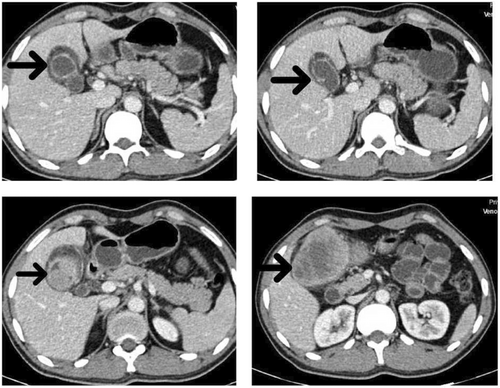
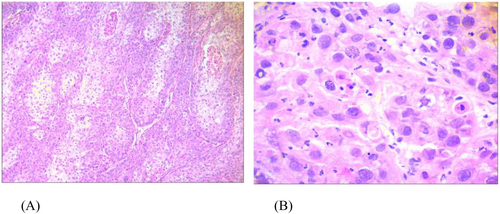
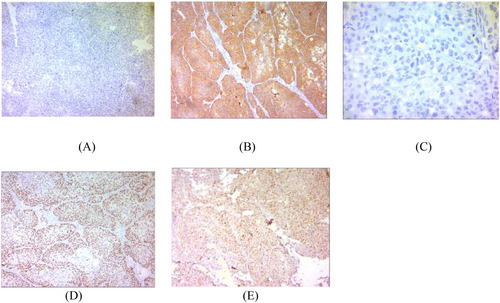
He was referred to a surgeon. On laparotomy, a liver parenchyma wedge biopsy was sent for rapid pathology diagnosis, which was reported as poorly differentiated squamous cell carcinoma (Figure 2). The gallbladder mass was 7 cm in the greatest dimension with serous perforation and involvement of the colonic wall. Cholecystectomy, right hemicolectomy, hepatic wedge resection, and regional lymph node dissection were performed. All surgical margins were free. The microscopic examination showed neoplastic tissue composed of nests of atypical cells with a high nuclear-cytoplasmic ratio and pleomorphism. Immunohistochemical staining was performed to confirm the diagnosis. The IHC (immunohistochemistry) study was positive for p63, CK7, and PanCK, up to 70% for Ki-67, and negative for chromogranin, synaptophysin, Heppar, and SALL4 (Figure 3).
2.2 Outcome and Follow-Up
Based on these findings, the diagnosis was compatible with poorly differentiated squamous cell carcinoma of the gallbladder (stage: T3N0M0). After surgery, he was referred to a radiation oncologist.
According to National Comprehensive Cancer Network (NCCN) recommendations, chemoradiation is a treatment option for this type of cancer but considering the patient's performance status the radiation oncologist prescribed six courses of adjuvant chemotherapy with FOLFOX (5FU 600 IV bolous then 3.5 g 5FU over 46 h with 600 mg leucoverin and 120 mg oxaliplatin). No dose reduction was needed during therapy.
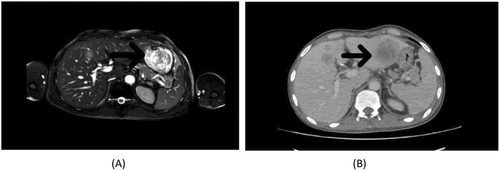
Two months following the end of adjuvant chemotherapy, magnetic resonance imaging of the abdomen with and without contrast injection was done, and non-adjacent liver metastasis was diagnosed.
The chemotherapy regimen consists of gemcitabine, cisplatin, and pembrolizumab started with every 3 weeks intervals, Following three courses of chemotherapy injections (9 weeks totally), the patient was reevaluated by abdominopelvic computed tomography and was reported as having a stable disease (Figure 4).
3 Discussion
Gall bladder cancer is the fifth most malignant tumor of the gastrointestinal tract. One of the important risk factors for gallbladder cancer is cholelithiasis [6].
Squamous cell carcinoma of the gallbladder is believed to arise from the basal cell layer of the epithelium. It can also result from squamous cell metaplasia or squamous cell carcinoma differentiation from a pre-existing adenocarcinoma. Chronic irritation from gallstones can lead to the differentiation of gallbladder glandular cells into metaplastic squamous epithelial cells, which undergo malignant transformation [1].
Gallbladder squamous cell carcinomas are characterized by a high proliferation rate and local invasiveness. Therefore, patients with advanced-stage gallbladder squamous cell carcinomas are diagnosed with a large tumor and involvement of nearby organs [7].
Gallbladder squamous cell carcinoma has the worst prognosis of all histological subtypes, with a median survival time of 7 months and a 5-year survival rate of less than 12% [2]. Squamous cell carcinoma of the gallbladder is a malignant tumor characterized by rapid proliferation and early spread to local and distant sites [8].
Due to the lack of a serous layer between the gallbladder and the liver, it can invade the liver parenchyma [9]. The most important treatment method is surgery. However, the reported resectability rate for these tumors was approximately 50% [10].
One of the most prognostic factors is tumor extension. If the tumor is limited to the subserosa and adjacent organs, the 5-year survival rate is 70% and 0%, respectively [11].
Ultrasound is usually the first diagnostic modality, but a definitive diagnosis is confirmed by histology [12]. CT scan and MRI should be carried out to rule out metastasis and plan the surgery [5]. In early detection of gall bladder squamous cell carcinoma, surgery is a curative treatment and can improve the prognosis [13].
Simple cholecystectomy is the standard modality for patients with Tis stage (carcinoma in situ) or T1a (invading mucosa) stage. For patients at the T1b (invading muscular layer) stage or higher, radical resection, including cholecystectomy, limited segmental resection of the liver (segment IVb. V), and regional lymphadenectomy [14], should be considered. Most patients with gallbladder squamous cell carcinoma are locally advanced. Treatments such as chemotherapy, radiotherapy, immunotherapy, and conservative care are appropriate [13].
The benefit of adjuvant therapy in patients with high-risk features, including positive nodes and incomplete resection, is reported in many studies. Still, it is unclear which of the treatments, chemotherapy or radiotherapy, is better. The NCCN recommends chemoradiotherapy [15]. In this case report, the patient was treated with surgery (cholecystectomy, wedge resection of liver, and right hemicolectomy) and six courses of adjuvant chemotherapy, and due to liver metastasis in follow up CT scan, he received four courses of gemcitabine, cisplatin, and pembrolizumab chemoimmunotherapy.
Author Contributions
Sahar Dashti: conceptualization, data curation, funding acquisition, project administration, writing – original draft, writing – review and editing. Elahe Mirzaee: data curation, formal analysis, writing – review and editing. Maryam Garousi: data curation, formal analysis, writing – original draft. Mastaneh Sanei: conceptualization, project administration, writing – review and editing. Navid Abdi: data curation, writing – original draft.
Acknowledgments
The authors acknowledge the support of Dr. Khodakarim in preparing the case report.
Ethics Statement
We obtained a written statement of informed consent from the patient for the publication of case details and the use of images. The case discussed in this manuscript does not include patient-identifying information, nor does it report a new study that required IRB approval.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data can be obtained from the corresponding author upon request.




