Reversible Cerebral Vasoconstriction Syndrome Following Blood Transfusion in a Patient With Chronic Anemia: A Case Report
Funding: The authors received no specific funding for this work.
Amogh Verma and Govind Sharma contributed equally and are the co-first authors.
ABSTRACT
Reversible cerebral vasoconstriction syndrome (RCVS) is a rare neurological disorder characterized by transient constriction and dilation of cerebral arteries, leading to severe headaches and neurological deficits. This case report describes a 41-year-old woman with chronic anemia, acute chronic kidney disease, type 2 diabetes mellitus, and rheumatoid arthritis who developed RCVS following transfusion of packed red blood cells (PRBCs). She experienced sudden-onset seizures and a thunderclap headache 5 days post-transfusion. Diagnostic imaging, including computed tomography (CT) and magnetic resonance imaging (MRI), revealed the characteristic features of vasogenic edema. The patient was treated with blood pressure control and symptomatic relief for pain, resulting in gradual improvement. This case highlights the importance of recognizing RCVS as a potential complication of blood transfusions, particularly in patients with significant comorbidities. Understanding the possible mechanisms, including rapid hemoglobin correction, and the effects of residual plasma and storage lesions in transfused blood, is essential to prevent and manage this rare but serious condition.
Summary
- Reversible cerebral vasoconstriction syndrome (RCVS) can occur following blood transfusion, emphasizing the need for vigilance in patients presenting with severe headache and neurological deficits post-transfusion.
- Distinguishing RCVS from other causes of thunderclap headache, such as subarachnoid hemorrhage (SAH), is critical for appropriate management.
1 Introduction
Reversible cerebral vasoconstriction syndrome (RCVS) is an uncommon but significant neurological disorder characterized by transient constriction and dilation of cerebral arteries, leading to severe headache, and various neurological deficits [1]. While RCVS is known for its hallmark symptom of thunderclap headache, it is crucial to distinguish it from subarachnoid hemorrhage (SAH), which can present similarly but requires different management strategies [2-4]. RCVS is often associated with triggers such as vasoactive drugs, postpartum states, and unruptured cerebral aneurysms, yet its occurrence following blood transfusion is notably rare [5-8]. The syndrome demands prompt recognition and intervention to mitigate potential complications, including seizures, visual disturbances, and hypertensive encephalopathy [8].
The pathophysiological mechanisms that trigger RCVS remain poorly understood in the context of blood transfusions, particularly in patients with chronic anemia. Rapid correction of hemoglobin (Hb) levels and changes in blood volume and viscosity may contribute to cerebral vascular dysregulation, leading to the onset of RCVS [9]. Additionally, factors such as the amount of residual plasma in packed red blood cells (PRBC) units and the duration of blood storage can influence vasoregulatory responses due to the accumulation of bioactive substances during storage [10, 11]. The recognition of RCVS in patients receiving blood transfusions is critical, given the potential for delayed symptom onset and the need for timely diagnostic imaging to confirm the condition [8]. Magnetic resonance imaging (MRI) and computed tomography (CT) scans play a pivotal role in identifying the characteristic features of vasogenic edema and excluding other potential causes of neurological symptoms [12]. Moreover, the management of RCVS requires a multidisciplinary approach, including blood pressure control, seizure management, and symptomatic relief of pain [1].
This case report highlights the clinical presentation, diagnostic complexities, and treatment approaches for managing RCVS following transfusion of PRBCs in a patient with chronic anemia, acute chronic kidney disease, type 2 diabetes mellitus, and rheumatoid arthritis. It emphasizes the importance of considering RCVS when evaluating patients who develop severe headache and neurological deficits after blood transfusion. Healthcare providers can enhance their ability to anticipate and manage these rare yet significant complications by understanding the potential triggers and underlying pathophysiological mechanisms. Insights from this case contribute to the expanding knowledge base on RCVS, stressing the necessity for vigilance and timely intervention in vulnerable patient groups.
2 Case Presentation
2.1 Case History and Examination
A 41-year-old woman with a history of chronic anemia secondary to menorrhagia due to uterine fibroids, acute exacerbation of chronic kidney disease, type 2 diabetes mellitus, and rheumatoid arthritis was admitted to a tertiary care center in Hapur, Uttar Pradesh, India, because of profound fatigue and dizziness. Her baseline Hb level was approximately 8.5 g/dL. However, recent laboratory tests showed a sharp drop to a critical level of 1.6 g/dL, which was attributed to acute blood loss exacerbating her chronic anemia. She was promptly transfused with five units of PRBCs over a 24-h period, resulting in an increase in her Hb to 8.7 g/dL. The PRBC units transfused were standard, non-leukoreduced units, with an estimated residual plasma volume of approximately 30 mL per unit. The blood products were stored for an average of 25 days, which is well within the acceptable storage duration. There was no clinical or laboratory evidence of hemolysis during or after transfusion, as indicated by stable bilirubin levels, absence of hemoglobinuria, and normal lactate dehydrogenase levels.
Simultaneously, transvaginal and pelvic ultrasonography revealed a uterine fibroid, prompting the initiation of medroxyprogesterone acetate 10 mg orally once daily to address hormonal imbalances associated with perimenopause. Five days later, she presented again at our institution in Hapur, experiencing sudden-onset seizures and a persistent, severe headache, as reported by her family. The seizures manifested as generalized tonic–clonic episodes with deviation of her right eye. Immediate treatment was initiated with an intravenous loading dose of levetiracetam 2000 mg over 15 min, followed by maintenance dosing of 1000 mg IV every 12 h. Following levetiracetam, intravenous lorazepam 4 mg was administered according to the status epilepticus protocol. Despite initial interventions, the seizures continued, leading to the administration of an intravenous loading dose of fosphenytoin at 20 mg PE/kg (1400 mg PE), which effectively halted the seizure activity. On examination, she was afebrile but hypertensive (170/90 mmHg), nonverbal, and experienced impaired vision. Neurological assessment revealed heightened muscle tone in the upper limb flexors and lower limb extensors without discernible focal deficits. Deep tendon reflexes were normal upon comprehensive evaluation.
2.2 Differential Diagnosis and Investigations
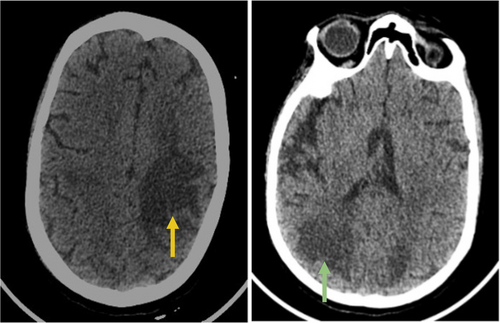
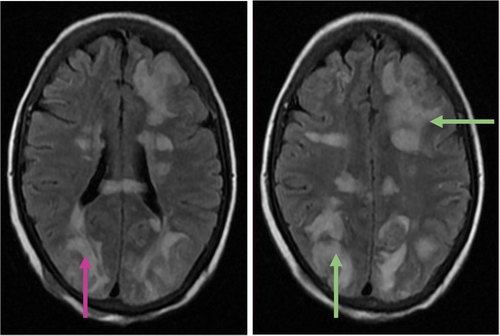
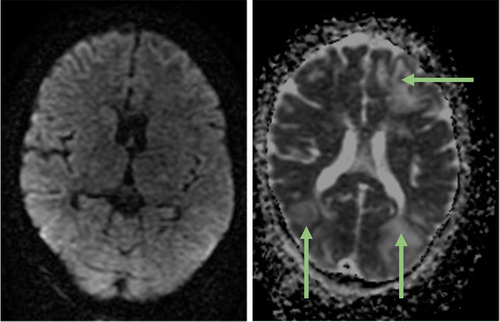
Differential diagnoses included SAH, intracerebral hemorrhage, posterior reversible encephalopathy syndrome (PRES), metabolic encephalopathy, and central nervous system infection or inflammation. Metabolic assessment revealed an elevated lactic acid level of 9.8 mmol/L, resulting in anion gap metabolic acidosis (pH 7.21). Screening for infections and toxins yielded negative results. Head CT imaging revealed hypodense regions in the subcortical areas of both cerebral hemispheres, suggesting vasogenic edema (Figure 1). Subsequent MRI with contrast using a T2-weighted/Fluid Attenuated Inversion Recovery (T2-FLAIR) sequence confirmed vasogenic edema in the deep white matter and subcortical regions, exhibiting a parieto-occipital pattern (Figure 2). Diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) sequences demonstrated increased diffusion on DWI and bright signals on ADC, consistent with vasogenic edema rather than cytotoxic edema (Figure 3). Stroke was ruled out owing to the absence of diffusion restriction on DWI, and non-contrast CT excluded SAH by revealing no evidence of hemorrhage.
Magnetic resonance arteriography and venography revealed no signs of venous sinus thrombosis or large artery occlusions. Treatment commenced with an intravenous nicardipine infusion starting at 5 mg/h, titrated up to 15 mg/h to address raised blood pressure. Alongside nicardipine, acyclovir was administered at 10 mg/kg IV every 8 h for suspected meningoencephalitis. Broad-spectrum antibiotics, including vancomycin 15 mg/kg IV every 12 h and ceftriaxone 2 g IV every 12 h, were initiated for suspected meningoencephalitis. Admission to the neurocritical patient care unit ensured ongoing monitoring. Following admission, an immediate lumbar puncture was performed to exclude central nervous system infection or SAH. Cerebrospinal fluid (CSF) analysis revealed 4950 red blood cells per microliter, normal glucose and protein levels, and the absence of xanthochromia, indicative of a traumatic tap. CSF culture and viral polymerase chain reaction (PCR) studies were negative. Consequently, meningoencephalitis treatment was discontinued. Sudden, severe thunderclap headache and imaging findings strongly suggested RCVS. A RCVS2 score of 9 (indicating a single thunderclap headache episode, female sex, and vasoconstrictive trigger) strongly supported the diagnosis of RCVS. The patient's recent blood transfusion, rapid correction of anemia, and potential exposure to vasoactive substances present in the residual plasma or storage lesions of the PRBCs were considered likely triggers.
2.3 Treatment
Treatment focused on controlling blood pressure and managing symptoms. She received a nicardipine infusion to address elevated blood pressure. alongside pain management with acetaminophen 650 mg orally every 6 h and oxycodone 5 mg orally every 6 h, administered as needed. Seizure prophylaxis was maintained using intravenous levetiracetam.
2.4 Follow-Up
Over the course of 7 days, her mental state steadily improved; she became responsive to questions and followed commands appropriately. She did not experience any additional seizures and reported a reduction in the severity of her headache. Her visual disturbances were resolved. The blood pressure gradually stabilized, and nicardipine was tapered off. The patient was transferred from the neurocritical care unit to an acute rehabilitation facility for ongoing therapy. At the third-week follow-up, the patient reported complete resolution of headaches and no neurological deficits. A follow-up non-contrast CT scan of the patient's head showed a significant reduction in the previously observed hypodensities, indicating the resolution of vasogenic edema. Given the uncertainty surrounding the effectiveness of calcium channel blockers in managing prolonged cerebral vasoconstriction, the medications were discontinued.
3 Discussion
We report a unique presentation of RCVS following PRBCs transfusion in an anemic patient with renal disease, type 2 diabetes mellitus, and rheumatoid arthritis. The precise mechanism by which RCVS is triggered during blood transfusion remains unclear. However, literature suggests that chronic anemia and a rapid rise in Hb levels exceeding 5 g/dL are potential risk factors for developing RCVS post-transfusion [13]. Thunderclap headache, as observed in this patient, is a defining feature of RCVS and is characterized by sudden, severe head pain reaching maximal intensity within seconds to minutes [14]. While thunderclap headache is also a classic presentation of SAH, the absence of hemorrhage on imaging and CSF studies helped exclude SAH in this case. Differentiating between RCVS and SAH is critical, as the management and prognosis differ. Detection of recurrent headaches is important for accurate diagnosis, and research indicates that 82%–100% of patients experience recurring headaches within days of symptom onset, with many enduring three to four episodes [15]. The initial symptoms often include an intense, pulsating headache, which may be followed by seizures.
Seizures, though less common, have been noted as an initial symptom in 1%–17% of RCVS cases, and while RCVS can develop spontaneously, it is often linked to triggers such as vasoactive drug use (including adrenergic and serotonergic agents), unruptured cerebral aneurysms, dissections of carotid-cervical or vertebral arteries, the postpartum period, and PRES, which shares overlapping radiological and clinical features with RCVS, suggesting a common underlying pathophysiology [14]. In PRES, impaired blood pressure autoregulation leads to vasogenic edema due to disruption of the blood–brain barrier and cerebral hyperperfusion [16]. Similarly, RCVS involves temporary dysfunction of vascular tone in the brain, causing multiple regions of cerebral arterial dilation and constriction [17]. Blood transfusion reactions typically are not considered to involve the brain. However, transfusion-related complications such as transfusion-associated circulatory overload (TACO) and transfusion-related acute lung injury (TRALI) have been well documented [18-20]. TACO results from rapid transfusion of blood products, leading to volume overload, while TRALI is thought to stem from immune-mediated reactions involving donor anti-leukocyte antibodies [21-23]. In the context of RCVS following blood transfusion, the pathogenesis may involve rapid changes in blood viscosity and volume, leading to cerebral autoregulatory dysfunction.
Factors such as residual plasma in PRBC units and the accumulation of bioactive substances during storage (known as storage lesions) may contribute to vasoregulatory changes [24]. The residual plasma may contain cytokines and other vasoactive mediators that can influence endothelial function [25]. Additionally, longer storage durations of PRBCs can lead to the accumulation of substances such as free Hb and microparticles that can affect vascular tone [26, 27]. In our case, the PRBC units had an average storage duration of 25 days, and while within acceptable limits, the possibility of storage lesions contributing to the patient's condition cannot be excluded. The immediate correction of severe anemia, resulting in a rapid increase in Hb from 1.6 to 8.7 g/dL, may have disrupted cerebral autoregulation. Patients with chronic anemia often have compensatory cerebral vasodilation to maintain adequate oxygen delivery [28]. Rapid correction can lead to sudden vasoconstriction, potentially triggering RCVS [8]. This mechanism is analogous to “misery perfusion” observed in patients with chronic carotid artery occlusions [28, 29].
Acharya et al. documented a case in which a patient with iron-deficiency anemia experienced a 5 g/dL increase in Hb level and developed RCVS 2 weeks later [30]. This parallels our patient's case, where symptoms emerged 5 days post-transfusion, indicating that symptom onset may be delayed. Factors contributing to this delay could include the quantity of blood transfused, the rate of transfusion, and the time needed for bioactive substances from residual plasma or storage lesions to exert their effects. MRI and CT are essential for prompt diagnosis. CT scans may reveal symmetrical vasogenic edema, whereas MRI, being more sensitive, shows T2-FLAIR hyperintensities in a parieto-occipital distribution. Diffusion-weighted magnetic resonance imaging confirmed vasogenic edema with no diffusion restriction. In our case, the imaging findings were consistent with vasogenic cerebral edema associated with RCVS. Reversible cerebral edema occurs in approximately 8%–38% of RCVS patients and typically resolves within 1 month, preceding the reversal of vasoconstriction [14].
Since RCVS is a rare neurological complication that can occur after blood transfusion, it is important to closely monitor patients for increases in blood pressure, headaches, and other neurological symptoms in the days following the transfusion. Clinicians should be aware of the potential for the delayed onset of symptoms. Early recognition and management are crucial to prevent complications, such as stroke or intracerebral hemorrhage.
4 Conclusion
This case highlights the occurrence of RCVS following blood transfusion in a patient with chronic anemia and multiple comorbidities. Rapid correction of severe anemia, residual plasma in transfused PRBCs, and storage lesions may have contributed to cerebral vasoregulatory dysfunction, leading to RCVS. Clinicians should be vigilant of neurological symptoms in patients receiving blood transfusions, especially those with significant anemia, and comorbidities. Further research is needed to understand the mechanisms linking blood transfusion to RCVS and to develop strategies for its prevention and management. Early recognition and appropriate management can lead to favorable outcomes, as demonstrated in this case.
Author Contributions
Amogh Verma: conceptualization, project administration, writing – original draft, writing – review and editing. Govind Sharma: conceptualization, project administration, writing – original draft, writing – review and editing. Ajeet Singh: writing – original draft, writing – review and editing. Harshit Gupta: writing – original draft, writing – review and editing. Deependra Pratap Singh: writing – original draft, writing – review and editing. Abhay M. Gaidhane: writing – original draft, writing – review and editing. Mahalaqua Nazli Khatib: writing – original draft, writing – review and editing. Ganesh Bushi: writing – original draft, writing – review and editing. Sanjit Sah: writing – original draft, writing – review and editing. Rodrigue Ndabashinze: writing – original draft, writing – review and editing.
Acknowledgments
The authors extend their sincere gratitude to Medicos In Research, Nautanwa, UP 273164, India, for their unwavering support and guidance throughout the manuscript development process. The authors also express their appreciation to the clinical team and clinicians managing the patient, particularly the Department of Radiology at Rama Medical College Hospital and Research Centre, Hapur, India, for their invaluable contributions and assistance. Additionally, the authors thank the patient and their family members for their cooperation in bringing this case to the scientific community.
Ethics Statement
Permission of the Institutional Ethics Committee (IEC) was waived because the study was a case report.
Consent
Written informed consent was obtained from the patient for publication of this case report, including accompanying images.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study. All the information is available within the manuscript (including images).




