Combination of bolus and continuous infusion of factor VIII in a patient with severe hemophilia A undergoing on-pump coronary artery bypass graft surgery
Key Clinical Message
Patients with hemophilia A can safely undergo cardiac surgery. Combining continuous infusion and boluses of factor VIII is both safe and cost-effective. Interdisciplinary approach and meticulous planning are crucial in the prevention of bleeding complications.
1 INTRODUCTION
Hemophilia A is an X-linked recessive disorder caused by a deficiency in factor VIII (FVIII). It affects 1/5000 males worldwide and has a prevalence of 173,711 cases worldwide, according to the 2018 survey of the World Federation of Hemophilia.1 With better therapy and increasing availability of coagulation factors, individuals with hemophilia live longer, with an estimated median life expectancy of 77 years in one Dutch study.2 Therefore, they are more likely to develop coronary artery disease. Therefore, on-pump cardiac surgery in patients with hereditary bleeding disorders is a special and challenging situation, and the evidence regarding optimal substitution regimes remains limited.
In this report, we would like to present a successful case of on-pump coronary artery bypass graft (CABG) surgery in a patient with severe hemophilia A. The patient gave his consent for publication.
2 CASE HISTORY
An 80-year-old, 72 kg, 175 cm male patient was referred to our center in December 2023 for a CABG surgery. He had a severe form of hemophilia A with FVIII activity of <0.5% and with no known anti-FVIII neutralizing antibodies.
3 METHODS
In collaboration with the patient's hemostaseologist, we devised a substitution strategy that incorporated intravenous boluses (IVB) and continuous infusion of FVIII, as depicted in Figure 1.

According to the hemostaseologist's recommendation, we administered an IVB of 4000 IU FVIII preoperatively, followed by a continuous infusion of 200 IU per hour. As an adjuvant, we added 1 g tranexamic acid IV.
Intraoperatively, we administered 500 IU/kg heparin, according to our standard regimen. During the cardiopulmonary bypass (CPB), we performed a point-of-care rotational thromboelastometry (ROTEM, Figure 2A) using ROTEM Sigma (Werfen, Barcelona, Spain). It showed a generalized clotting factor deficiency, most probably due to the effect of hemodilution. The clot firmness, however, was within the normal range. We decided to administer 3000 IU of 4-factor prothrombin complex concentrate (PCC) after the termination of the CPB, guided by clotting time (CT) in the EXTEM assay of ROTEM. The patient received two grafts: left internal mammarian artery to the left anterior descending artery (LIMA-LAD) and a saphenous vein graft to the second marginal branch of circumflex artery (M2).
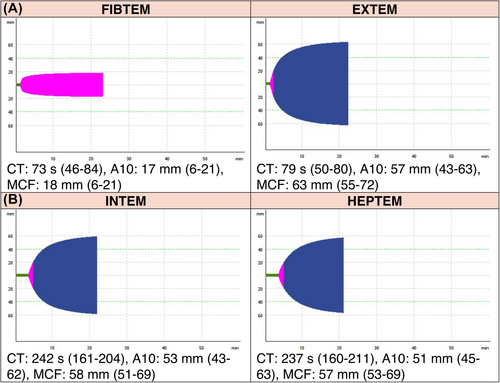
After the weaning from the CPB and administration of PCC and 392 mg protamine, based on our standard institution policy, we repeated the ROTEM (Figure 2B). The activated clotting time (ACT) with and without heparinase addition, as measured with Medtronic ACT Plus (Medtronic, Dublin, Ireland), resulted in 154 s in the heparinase channel and 164 s in the native channel.
Upon closing the chest, the cardiac surgeon deemed the hemostasis to be excellent and estimated the blood loss during the surgery to be 200 mL.
In the ICU, we continued the continuous infusion at the same rate as in the operating theater. The cumulative blood loss in the ICU was 500 mL. Further postoperative course was uneventful, and the hemodynamically stable patient could be transferred to the ward on the postoperative day (POD) 1.
4 CONCLUSION AND RESULTS
In the following days, we continued the FVIII substitution according to the preoperative plan. The patient received 43,000 IE FVIII in total. He was discharged on POD 15 without any bleeding complications.
5 DISCUSSION
Based on FVIII activity, hemophilia A is classified as mild (5%–40%), moderate (1%–5%), and severe (<1%).3 Patients with severe disease are at risk of spontaneous bleeding.
1 IU of FVIII is equivalent to the amount present in 1 mL of plasma from a healthy individual.6 The half-life (t1/2) varies between 5 and 22 h, with a mean value of 12 h, but it may also be agent-specific.6 Our patient was known to have t1/2 close to this mean value.
Some authors recommend administering FVIII as a continuous infusion. They claim that it improves safety, reduces transfusions, and major bleeding complications.7, 8
We decided to administer 50 IU FVIII/kg, followed by the infusion of 2.5 IU FVIII/kg/h to achieve FVIII activity at a safe level of above 90% on POD1. The theoretical FVIII activity, assuming a t1/2 of 12 h and no influence of cardiopulmonary bypass, is shown in Figure 3A (dashed line). Alternatively, the goal of achieving a constant FVIII activity of 90% (solid line) can be met by administering 45 IU FVIII/kg, followed by the infusion of 5 IU FVIII/kg/h. Interestingly, following this strategy will result in a cumulative dose of 165 IU/kg FVIII instead of 110 IU/kg in the presented case, even though the FVIII activity is constantly lower.
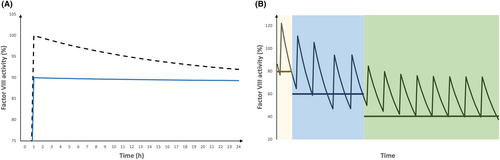
After terminating the continuous infusion on POD1 in the afternoon, the following doses were administered as IVB (25 bid and 18.75 IU/kg bid on POD2-3 and POD4-7, respectively). The theoretical FVIII activity on days 1–7, assuming a half-life of 12 h, is presented in Figure 3B.
Although we decided to administer FVIII as a continuous infusion, many authors have managed their patients with IVB only. In their review of published cases, Lin and Yao identified 27 cases treated with IVB, 24 of them with no major bleeding complications.9
Viscoelastic methods, like thromboelastography (TEG) or rotational thromboelastometry (ROTEM) are becoming increasingly available. Recently, there have been several publications regarding its usefulness in providing care for patients with hemophilia.10, 11 As a bedside examination, it may be especially useful in the centers, which do not have immediate access to FVIII activity testing. However, Misgav et al. proved that the administration of both heparin and protamine affects the FVIII activity. Heparin, at concentrations of 2–4 μm/mL (which is typical for the on-pump surgery), inhibits FVIII.11 An excessive concentration of protamine has a similar effect. Therefore, a careful titration of protamine is advised.11 This has also been shown to be better achieved with viscoelastic tests than with ACT.12
Whether the CT value in on-pump HEPTEM (which adds heparinase to inhibit the circulating heparin) provides adequate information about FVIII activity remains unclear. In our case, the difference between on-pump and post-protamine HEPTEM-CT might be explained by the effect of PCC, which contains factors II, VII, IX, and X.13 Even though the substitution of PCC is guided by CT values in the EXTEM, the presence of administered factors IX and X may interfere with coagulation times in the INTEM assay.
In conclusion, cardiac surgery in patients with hereditary coagulation disorders is feasible. Nevertheless, an interdisciplinary collaboration of ambulant hemostaseologists, cardiac surgeons, and cardiac anesthetists is strongly advised.
AUTHOR CONTRIBUTIONS
Stanislaw Vander Zwaag: Conceptualization; visualization; writing – original draft; writing – review and editing. Stefan Brose: Conceptualization; writing – original draft; writing – review and editing. Jens Fassl: Conceptualization; supervision; writing – original draft; writing – review and editing.
ACKNOWLEDGMENTS
We would like to thank Annette Haenel, MD, for helping us develop the perioperative substitution plan.
FUNDING INFORMATION
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sector.
CONFLICT OF INTEREST STATEMENT
The authors declare there are no conflicts of interest.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
Data might be obtained from the corresponding author upon a reasonable request.




