Unusual coexistence: A case of mixed small and large cell neuroendocrine tumor in a bladder previously affected by bilharzial cystitis
Key Clinical Message
Neuroendocrine tumors of the bladder are rare, accounting for less than 1% of all bladder tumors. Among these, large cell neuroendocrine carcinoma is an extremely uncommon subtype. We report on a histologically confirmed case of mixed both large and small cells neuroendocrine tumor of the bladder in a 64-year-old male with a history of bilharzial cystitis. The diagnosis was made after radical cystectomy with Immunohistochemical staining revealing positivity for synaptophysin, CD56, and AE1/3. While bilharzia is commonly linked to squamous cell carcinoma in the bladder, the potential relationship with neuroendocrine tumors is still relatively unexplored in this context. This case marks the first reported instance of the atypical coexistence of bilharzial cystitis and mixed large and small cell neuroendocrine carcinoma of the bladder. This unique case of coexisting highlights a rare phenomenon warranting further study. Similar associations have been documented in other organs, emphasizing the importance of exploring underlying mechanisms and clinical implications for improved patient care and outcomes.
1 INTRODUCTION
Neuroendocrine neoplasms originating in the urinary bladder are exceptionally uncommon. According to the latest WHO/ISUP classification from 2016, there are four recognized types of neuroendocrine neoplasms found in the urinary bladder: small cell neuroendocrine carcinoma, large cell neuroendocrine carcinoma, well-differentiated neuroendocrine tumor, and paraganglioma. Small cell neuroendocrine tumors make up less than 1% of malignant bladder tumors, while large cell neuroendocrine tumors represent an even rarer subset.1-3
In bilharzia-endemic areas, schistosomiasis is a common infection, and it is recognized as a risk factor for squamous cell carcinoma of the bladder. Research indicates that schistosomiasis has also been linked to various other histotypes, such as neuroendocrine tumors of the gastrointestinal tract.4, 5 Admixing of both small cell neuroendocrine and large cell neuroendocrine tumors is rare. However, such a coexistence has been described in a very few patients, but it has not been linked to schistosomiasis.6, 7
2 CASE HISTORY AND EXAMINATION
A 64-year-old male patient with a history of bladder cancer diagnosed in Zimbabwe sought further medical care and treatment in South Africa. Initially, the patient presented with painless hematuria accompanied by clots. The patient is a non-smoker with no relevant occupational exposures. He had previously been treated for bilharzia during childhood and has a known history of diabetes and hypertension, managed with insulin and two antihypertensive medications. Past surgical history includes three TURBT procedures in Zimbabwe with the first one, revealing low-grade papillary urothelial carcinoma with crush artifact limiting diagnostic evaluation, detrusor not identified in specimen, a second TURBT 3 months later showing chronic cystitis with no dysplasia or malignancy and presence of schistosomiasis, and a third TURBT 6 months after the last one displaying invasive moderately differentiated urothelial cancer with detrusor not identified and presence of schistosomiasis in specimen.
Clinically, the patient appeared not acutely or chronically unwell with no abnormalities noted on general examination. Abdominal examination did not reveal any palpable masses. Additionally, there were no abnormalities detected on respiratory, cardiovascular, or central nervous system examination. Digital rectal examination showed a benign prostate and a palpable mobile bladder mass on bimanual examination.
3 DIAGNOSIS, INVESTIGATIONS, AND TREATMENT
In terms of preoperative work up, blood results showed within normal range electrolytes, renal function tests, liver function tests, lipid profile, inflammatory markers, PSA, Troponin T, HbA1c, and NT-proBNP.
Imaging studies included a chest x-ray and KUB x-ray which showed no abnormal findings. A CT scan also performed revealed a lobulated heterogeneous enhancing urinary bladder mass measuring 6.4 × 6.2 cm (Figure 1A,B), an enlarged prostate measuring 6.8 × 6 × 6.5 cm, a Bosniak 1 cyst in the left kidney, pelvic lymph node enlargement, femoral lymph node enlargement on both sides, and spondylosis of the thoracic and lumbar spine.
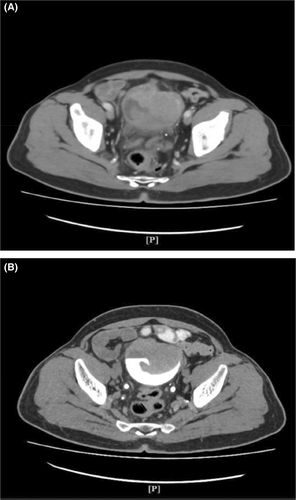
A radical cystoprostatectomy and ileal conduit, along with standard template of pelvic lymph node dissection. The histology report reveals a diagnosis of neuroendocrine carcinoma—T2B, involving the entire bladder and comprising a large cell component (20%) and a small cell component (80%) with immunohistochemical staining revealing positivity for synaptophysin, CD56, and AE1/3.
The tumor extends into the deep muscle propria, with no involvement of the prostate or soft tissue, while lymph-vascular invasion is present there was no lymph nodes metastases out of all the 15 nodes retrieved.
4 OUTCOME AND FOLLOW-UP
The immediate postoperative period was uneventful, with no complications. Based on the histological findings, the patient was referred to the medical oncology team for assessment of potential chemotherapy, but the patient opted to return home in Zimbabwe and was lost to follow-up. While there, lung and liver metastases were diagnosed 9 months later. Following this, the patient succumbed to the cancer in Zimbabwe after a failed chemo regimen while arrangements were being made for their transfer back.
5 DISCUSSION
Primary neuroendocrine tumors of the bladder are very uncommon, representing less than 1% of bladder tumors. Among the four types of neuroendocrine tumors recognized in the WHO/ISUP 2016 classification, small cell is the most common, whereas large cell is extremely rare. However, small or large cell neuroendocrine tumors can be pure or mixed with urothelial carcinoma or other histological types.1, 2, 7 In this case, both types were present, with a large cell component comprising approximately 20%.
It is important to note that our patient developed a bladder tumor on a bladder previously affected by bilharzial cystitis. This unique case not only showcases a rare histological type of bladder tumor but also highlights the uncommon coexistence of bilharzia, which is often associated with squamous cell carcinoma. Existing literature has established an association between bilharzia and the development of neuroendocrine tumors in the gastrointestinal tract.4 It is probable that chronic inflammation from bilharzial cystitis played a role in the development of neuroendocrine tumors in our patient's bladder.
From a histological perspective, large cell neuroendocrine carcinoma consists of large polygonal tumor cells exhibiting abundant cytoplasm, prominent nucleoli, and high mitotic activity. It displays growth patterns including solid, trabeculated, nested, pseudoglandular, organoid, and palisading structures.8
Conversely, small cell neuroendocrine carcinomas are characterized by sheets or nests of small to intermediate cells with a high nuclear-to-cytoplasmic ratio, molding, scant cytoplasm, inconspicuous nucleoli, and evenly dispersed “salt and pepper” chromatin.8
Immunohistochemical staining plays a crucial role in confirming the impression of the light microscopic appearance of both small and large cell carcinomas. Markers that are typically expressed include CD56, synaptophysin, chromogranin A, and AE1/3. Our case demonstrated positivity for synaptophysin, CD56, and AE1/3 consistent with neuroendocrine differentiation.1, 2, 6-9
Both small cell and large cell neuroendocrine tumors are aggressive and can present either as localized disease, as is the case in our patient, or as metastatic disease. However, since large cell neuroendocrine carcinoma is rare, its biological and clinicopathologic characteristics are largely unknown. This lack of understanding hinders the development and assessment of effective therapeutic approaches. Existing knowledge about this disease is restricted, primarily derived from small series and case reports. As of now, there is no established consensus on the standard treatment for patients affected by this aggressive malignancy.7, 8 A study at a single center found a difference in disease-free survival rates between five patients treated with adjuvant chemotherapy versus surgery alone, but the small number of cases limited the conclusiveness of the finding. Chemotherapeutic regimens are often adapted from those used for pulmonary neuroendocrine tumors, making neoadjuvant or adjuvant combination etoposide and platinum-based therapy the preferred treatment option. While surgery alone is not advised, it remains crucial in the comprehensive care of these patients.8
Establishing a standard treatment guideline for small cell neuroendocrine carcinoma is also challenging due to its rarity. Patients are classified into those with localized or metastatic disease. For patients with localized disease, systemic chemotherapy followed by either radical cystectomy or radiation therapy is suggested. This approach found that patients undergoing preoperative chemotherapy had better cancer-specific survival rates compared to those who underwent immediate cystectomy (78% vs. 36%). Due to the absence of specific guidelines for small cell carcinoma, most chemotherapy regimens are adapted from protocols used for small cell lung cancer. Typically, a platinum-based chemotherapy agent is combined with etoposide. While cisplatin is recommended, carboplatin can be considered as an alternative if cisplatin is contraindicated or unavailable.7-9 Based on this literature review, our patient was referred to the medical oncology team for assessment and potential adjuvant chemotherapy, as optimal treatment strategies remain uncertain.
In terms of prognosis, the pure form of large cell neuroendocrine cancer could possibly carry a worse prognosis than mixed forms based on a study with a small sample size by Wang et al. The worse outcome of pure large cell neuroendocrine carcinoma, compared with mixed types, could be due to the disease stage at the time of diagnosis.9 However, for these conditions, long-term follow-up is crucial for monitoring recurrence and disease progression.
6 CONCLUSION
The coexistence of bilharzial cystitis and mixed large and small cell neuroendocrine tumors in the bladder presents a rare and intriguing clinical scenario. While bilharzia is typically associated with squamous cell carcinoma in the bladder, emerging evidence from other organs suggests potential interactions between bilharzia infection and the development of neuroendocrine tumors. We strongly believe that the same mechanism involved in these interactions outside the bladder is implicated in our case. Further research is warranted to elucidate the underlying mechanisms driving this unique coexistence and to explore potential implications for diagnosis, treatment, and patient outcomes in affected individuals.
AUTHOR CONTRIBUTIONS
Mohammed Saleh E. Khalifa Salem: Methodology; writing – original draft. Abdul Alherek: Writing – original draft. Martin Van Rooyen: Writing – original draft. Alain Mwamba Mukendi: Conceptualization; data curation; formal analysis; methodology; writing – original draft; writing – review and editing.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




