Fatal case of meningococcal meningitis in a child from rural Bhutan: A case report
Key Clinical Message
N meningiditis remains an important cause of central nervous system infection. A high index of suspicion is required especially in infants. While empirical antibiotics may be initiated, diagnostic measures must be adopted for guided therapy. Notification of such cases contributes to surveillance data and deciding on providing vaccines to the population.
1 INTRODUCTION
Meningitis is a serious health condition that caused 236,000 deaths and 2.51 million incident cases globally in 2019.1 While there is a decrease in age-standardized mortality rate from 7.5 per 100,000 population in 1990 to 3.3 in 2019, bacterial meningitis remains a significant concern with 112,000 deaths and 1.28 million incident cases reported in children younger than 5 years.1 In South Asia, the mean annual incidence of bacterial meningitis among children was 105 per 100,000 up till 2017.2
Based on data from a surveillance network involving 58 countries between 2014 and 2019, a bacterial pathogen was identified in 2.6% of cases with suspected meningitis.3 The predominant bacteria detected in confirmed cases were Streptococcus pneumoniae (60.9%), Neisseria meningitidis (21.4%) and Haemophilus influenzae (17.7%).3 The highest proportions of total all-age meningitis deaths in 2019 were attributable to S pneumoniae (18.1%), N meningitidis (13.6%) and K pneumoniae (12.2%) with a significant decline in deaths due to H influenzae especially among children younger than 5 years.1 In the Southeast Asia region, surveillance data reports that 81.6% of confirmed bacterial meningitis were due to S pneumoniae3 while pooled estimates report that 13% of confirmed cases were due to H influenzae, 10% due to S pneumoniae and only 1% due to N meningitidis.2
The World Health Organization has adopted a global road map to eliminate epidemics of bacterial meningitis, reduce cases of vaccine-preventable bacterial meningitis by 50% and deaths by 70%, and reduce disability and improve quality of life after meningitis of any cause by 2030.4 This strategy calls for prevention and epidemic control, diagnosis and treatment, disease surveillance, providing support and care for people affected by meningitis, and advocacy and engagement. Therefore, it becomes important for countries to identify the burden of bacterial etiology and adopt evidence-based policies to reduce the disease burden or prevent outbreaks.
In this article, we present a report on meningococcal meningitis in a school boy from rural Bhutan. Bhutan is one small country located in the eastern Himalayas with a population of 0.7 million people with a crude birth rate of 15.5 per 1000 population and a general fertility rate of 57.3 per 1000 women of reproductive age (15–49 years).5 The country has a three-tiered free healthcare system with a neonatal mortality rate of 21 per 1000 live births6 and an under-five mortality rate of 34.1 per 1000 in 2017.5 Meningitis and/or encephalitis remains a significant problem with an average of 217 cases reported annually between 2017 and 2022.6 In 2022, there were 32 cases of meningitis reported among children <5 years and nine cases among children between ages 5 and 15 years.6 Among children 2–59 months admitted for pneumonia at the National Referral Hospital between 2017 and 2018, nasopharyngeal carriage of S pneumoniae was 62.8% including highly invasive serotypes 14, 3, and 1.7 In Bhutan, vaccination for Haemophilus influenzae b was introduced in 2009 as a part of the Pentavalent vaccine and pneumococccal conjugate vaccine (PCV13) in 2019 as a part of the routine immunization schedule leading to a decrease in the burden of these two agents. As of 2024, bacterial culture facilities are available only in four hospitals and polymerase chain reaction available only at the national reference laboratory. Therefore, there is paucity of aggregate information on the etiology of meningitis reported in the country. This case reports on a confirmed case of meningococcal meningitis in a school boy from rural area in Bhutan.
2 CASE PRESENTATION
2.1 Case history and examination
A ten-year-old, previously healthy child from Barshong, Sarpang district in Bhutan, who attended school during the day, returned home with fever, generalized body ache and joint pains. He developed high-grade fever towards the evening along with several episodes of watery diarrhea. The parents cared for the child at home until the early hours of the next morning when the child started to become increasingly drowsy and irritable. He was brought to the local hospital where he was found to have fever and purpuric rashes on his upper and lower limbs, irritability and restlessness (Figure 1). Following consultation with the on-call pediatrician with photographs of the rash, the child was immediately referred to the Central Regional Referral Hospital, Gelephu. The child received first dose of Ceftriaxone 1 g before referral. On arrival to the emergency room, the child was found to be restless and irritable along with poor perfusion and signs of shock. He was managed with oxygen supplementation, fluid bolus and was shifted immediately to the Pediatric Intensive Care Unit (PICU).
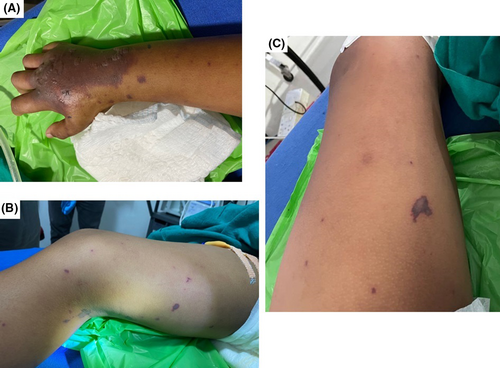
On arrival to the PICU, the child was restless, anxious and agitated, Glasgow Coma Scale (GCS) E4V3M5. He had a temperature 37.8°C, PR 118/min, BP 90/70 mmHg, RR 22/min, SpO2 99% on face mask, capillary refill time of 3 s, and weight 38 kg. He had no neck stiffness. He had scattered purpuric rashes with bullae on both upper and lower limbs (Figure 1).
2.2 Investigations
A preliminary investigation at the primary health centre showed a normal leucocyte count with a neutrophilic predominance (Table 1). Following admission, serial blood counts revealed neutrophilic leucocytosis with progressive thrombocytopenia. There was mild increase in urea/creatinine 61/1.6 mg/dL which improved with shock management. Tests for common locally prevalent tropical infections such as dengue, scrub typhus and malaria were negative. Lumbar puncture revealed a turbid cerebrospinal fluid (CSF) with 1450 leukocytes, predominantly lymphocytes and a low glucose. Gram stain and cultures from CSF and blood were negative. A contrast-enhanced computed tomography of the brain done prior to lumbar puncture revealed meningeal enhancement with no midline shift, hydrocephalous or cerebral infarcts.
| Test parameters | At local hospital (Day 1) | At referral centre (Day 1) | At referral centre (Day 3) | Normal range |
|---|---|---|---|---|
| WBC | 5600 | 10,130 | 26,930 | 4000–10,000 cells/cmm |
| Neutrophils (%) | 86 | 91 | 96 | |
| Lymphocytes (%) | 5 | 3 | ||
| Hemoglobin | 10.9 | 10.9 | 14.4 | 13–17 g/dL |
| Platelet | 100 | 93 | 65 | 150–450/cmm |
| AST/ALT | 80/37 | 5–40 U/L | ||
| Bilirubin | 0.4 | |||
| BU/SCr | 31/2.1 | 61/1.6 | 55/1.0 | <1.2 mg/dL |
| Na/K | 142/2.7 | 149/3.6 | 133–146 / 3.8–5.4 mEq/L | |
| Ca | 8.3 | 9.1 | 8.6–10.8 mg/dL | |
| C-reactive protein | 46.1 | 0–0.6 mg/dL | ||
| Scrub typhus IgM | Negative | Negative | ||
| Dengue NS1, IgM, IgG | Negative | Negative | ||
| Malaria parasite | Negative | |||
| Blood culture | No growth | |||
| Blister fluid culture | No growth | |||
| Urine analysis | Normal | |||
| Urine culture | No growth | |||
| Stool examination | No ova, cyst | |||
| Stool culture | No growth | |||
| Cerebrospinal fluid analysis | Appearance: Turbid | |||
| WBC: 1450/cmm | ||||
| Neutrophils: 10% | ||||
| Lymphocytes 90% | ||||
| Glucose: 39 mg/dL | ||||
| Protein 2.1 mg/dL | ||||
| LDH: 3113 U/L | ||||
| Gram stain: Negative | ||||
| Culture: No growth | ||||
Based on clinical presentation with fever, purpuric rash, and rapid progression to septic shock, a provisional diagnosis of meningococcal meningitis was made.
2.3 Case management
The report of CSF, whole blood and blister fluids that were tested 2 days later was positive for Neisseria meningitides on real time polymerase chain reaction conducted at the Royal Centre for Disease Control, Thimphu (Table 2). The identification of subtype of meningococcus could not be done due to lack of facilities in the country. Ceftriaxone 1 g q12h, which was started from the beginning was continued and intravenous Chloramphenicol 500 mg q6h was added. In view of the shock and hypoxia on arrival at the PICU, the child was intubated and ventilated. He required fluid boluses and inotropes (Dopamine and Noradrenaline) to maintain blood pressure although the BP was extremely labile requiring frequent titration of the inotropes. Due to persistent hypotension and poor perfusion despite inotropes and fluid administration, he received intravenous hydrocortisone to cover for a possible adrenal crisis. Despite aggressive shock management and ventilatory support, the child had persistent fever and his condition deteriorated with progressive decline of general condition and GCS to 2T. His pupils were dilated with absent corneal reflex by 3 h after admission. Understanding the grave condition and outcome, the family was counseled in detail on the diagnosis, management, condition of the patient and prognosis. The parents and family members were understanding of the situation and cooperated fully with the medical team.
| Specimen | Real time polymerase chain reaction | ||
|---|---|---|---|
| Streptococcus pneumoniae | Haemophilus influenzae | Niesseria meningiditis | |
| Cerebrospinal fluid | Negative | Negative | Positive |
| Blister fluid | Negative | Negative | Positive |
| Whole blood | Negative | Negative | Positive |
| Serum | Negative | Negative | Positive |
2.4 Outcome and follow-up
The child remained on life support for 12 days and succumbed to the illness. As the patient had absent brain stem reflexes from the first day of admission, the family signed the Do Not Attempt Resuscitation orders after discussing with the medical team and thus no cardiopulmonary resuscitation was performed. The patient attendants and staff who attended to this patient were given prophylaxis with Ciprofloxacin 500 mg single dose.
3 DISCUSSION
Patients with suspected bacterial meningitis or meningococcal diseases should be considered as an emergency and antibiotics should be delivered without delay.8, 9 The common symptoms of meningitis include fever, headache, neck stiffness and altered level of consciousness. A high index of suspicion is required for the diagnosis of meningitis in children. Additional symptoms include irritability, lethargy, poor feeding, unusual behavior, and bulging fontanelle. It is important to carefully evaluate for skin rash and petechiae after adequately exposing the patient. In patients with signs and symptoms of shock, the presence of nonblanching rash and purpura, strongly suggests the diagnosis of meningococcal meningitis; however, the absence of rash does not rule it out.8 N meningitidis is also associated with purpura fulminans that develop as erythematous macules on the trunk and extremities and progress to a livedo pattern and necrosis of dermis.10 Meningococcal sepsis is the predominant cause of Waterhouse–Friderichsen syndrome that presents as refractory shock, hyponatraemia and hyperkalemia that requires prompt diagnosis and adrenal function replacement.11 Travel history or involvement in close proximity in crowds is of importance to identify and contain outbreaks of meningococcal meningitis.
In addition to routine evaluation, evaluation of CSF and neuroimaging provides additional information about the possible etiology of bacterial meningitis. The detection of the causative agent in the CSF or blood using cultures, polymerase chain reaction, and whole genome sequencing helps provide guided therapy.9 N meningitidis is a gram-negative diplococcus that may be identified through culture and PCR techniques. A throat swab may be performed for meningococcal culture. In patients with meningococcal meningitis, it is important to evaluate for immunodeficiency status. Because N meningitidis is a capsulated organism, the incidence of invasive meningococcal disease is higher in those with complement deficiency such as C6 deficiency, C6 quantitatively 0 (C6Q0) or C9 deficiency.12, 13
The choice of antibiotic for N meningitidis is third-generation cephalosporin and penicillin.8, 9 However, there are concerns about resistance to quinolones, ceftriaxone, tetracycline, ampicillin, gentamicin, and amikacin reported in India.14 In N meningitidis, the most common cause of beta-lactam resistance is due to mutations in penicillin-binding proteins.13 In our case, parents and health staff who came in contact with the child were given prophylactic antibiotic. However, given the lack of adequate evidence regarding its effectiveness and concerns about rising resistance of N meningitidis, guidelines do not recommend it8 even during outbreaks.14
Meningitis is a life-threatening condition with high mortality related to sepsis and loss of cerebral auto-regulation. The risk of mortality is higher among children and in settings where there is a delay in presentation to the hospital, delay in diagnosis, or delay in the initiation of antibiotics. In many low- and middle-income countries, access to healthcare services and timeliness of interventions are directly related to the outcome of meningitis. The World Health Organization strategy for meningitis calls for diagonal approach with the integration of prevention and control measures into primary health care settings and optimizing diagnostic and treatment networks.15 This means the identification of patients that fit case definition of meningitis and timely referral to centres capable of performing diagnostic tests and providing appropriate management. With availability of appropriate critical care support, a case of meningococcal meningitis with clinical herniation from marked cerebral oedema has survived.16
Surveillance is critical in early detection and response to outbreaks of meningococcal disease.13 N meningitidis is found in the human nasopharyngeal mucosa, it's only natural reservoir. In countries in Africa, the pooled prevalence of carriage was 0.53% for NmA, 0.15% for NmW and 1.4% for NmX in endemic context.17 Among household contacts in Nepal, the carriage rate was 15% with average duration of 60 days.18 It is likely that the duration of carriage in contacts is longer if the index case has a more severe form of invasive meningococcal disease.18
Invasive meningococcal disease occurs as sporadic cases or in outbreaks that have been reported in neighboring countries. The last known outbreak of meningococcal meningitis in Bhutan was in 2010 at a boarding school with congested hostels that lacked adequate ventilation.19 The outbreak involved 19 cases and resulted in three deaths. Bacteriological confirmation was established in one of the cases that was referred to the National Referral Hospital while the other cases were managed at the district hospitals. Follow-up data from one of the survivors discharged from the National Referral Hospital reported hearing impairment on the right side. All other students and teachers were administered chemoprophylaxis with Rifampicin 600 mg q12h for 2 days as a part of the outbreak response. In 2016, a case of meningococcal septicaemia reported in a one-year-old patient at the National Referral Hospital.20 In this case, N meningitidis was isolated on blood culture and was found resistant to Ampicillin and Ceftriaxone.
To prevent meningococcal outbreaks, vaccination plays an important role. Countries in South Asia including Bhutan have adopted vaccination of S pneumoniae and H influenzae type b as a part of their national immunization programme. However, no country in South Asia offers routine vaccination for meningococcus given the relatively low burden of the disease. However, vaccination is recommended for those with clinical indications such as an immunodeficiency state or to contacts in events of outbreaks in India.14 Following events of outbreaks, South Korea has adopted quadrivalent vaccine (A, C, Y, and W-135) for all military recruits.21 At a population level, in countries that have adopted vaccination, there has been a significant decrease in the carriage rate of serogroups covered by the vaccine.22 However, development of protein-polysaccharide conjugate vaccine has been impeded by low immunogenicity against NmB.23 In Bhutan, meningococcal vaccine is offered only for those patients with specific indications/recommendations.
This case report highlights a sporadic case of meningococcal meningitis occurring in a schoolboy from rural Bhutan. Given that it resulted in mortality, it calls for a review of events that can prevent similar mortalities in the future in terms of early detection, providing appropriate level of care, identification of serogroups, and timely response to surveillance. One of the guiding policies of the WHO 2030 meningitis strategy recommends improving country-level capacity to prevent, diagnose, and respond to cases/outbreaks of meningitis. Meningitis/encephalitis syndrome are notifiable in Bhutan and should be reported through the National Early Warning, Alert Response and Surveillance System to the Royal Centre for Disease Control.
4 CONCLUSION
This was a sporadic case of bacteriologically confirmed meningococcal meningitis in Bhutan. Even though meningococcal meningitis is rarely reported in the country, this report highlights the clinical importance for active diagnostic efforts to provide guided-therapy to patients. In addition, this report serves as an important data point in the surveillance of meningococcal meningitis in Bhutan.
AUTHOR CONTRIBUTIONS
Purushotam Bhandari: Conceptualization; data curation; formal analysis; investigation; methodology; project administration. Thinley Dorji: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; writing – original draft. Tulsi Ram Sharma: Conceptualization; data curation; investigation. Mimi Lhamu Mynak: Conceptualization; data curation; investigation.
ACKNOWLEDGMENTS
We thank the parents of the patient for kindly providing consent to publish this case report. We thank the staff of the Departments of Emergency Medicine and Pediatrics, Central Regional Referral Hospital, Gelephu and the staff of the Royal Centre for Disease Control, Thimphu for their diligent support in the management of this case.
FUNDING INFORMATION
No funding available.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest.
ETHICS STATEMENT
A waiver for ethics review process was granted by the Institutional Review Board, Khesar Gyalpo University of Medical Sciences of Bhutan, Thimphu, Bhutan (IRB/Waiver-Exempt/PN-2024-015/1229 dated 28 June 2024) after the case report and patient consent were submitted. Written informed consent was obtained from the father for the publication of this case report and any accompanying images.
CONSENT
Written informed consent was obtained from the father for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Open Research
DATA AVAILABILITY STATEMENT
All relevant sources of the information are cited in this article.




