Congenital lymphatic dysplasia and severe bone disease in a term neonate with a novel homozygous PIEZO1 variant
Key Clinical Message
We report a patient with nonimmune fetal hydrops and multiple pathologic fractures. RNA analysis revealed a novel PIEZO1 variant. This report is the first to elucidate PIEZO1's role as a critical regulator of bone mass and strength.
1 INTRODUCTION
The PIEZO1 gene (PIEZO-Type Mechanosensitive Ion Channel Component 1; OMIM 611184) is located on chromosome 16 (16q24.3) and encodes the largest known human transmembrane protein, PIEZO1. The PIEZO1 protein has low tissue specificity, showing expression in at least 27 different tissue types.1 This mechanoreceptor is activated by mechanical force and controls potassium and calcium flow in multiple cell types, most prominently lung, bladder, skin, and red blood cells.1, 2 Studies have shown that in fetal tissue, the highest expression of this protein is in the liver, spleen, and lymphatic vessels.1, 2 PIEZO1 channels are crucial during the development of lymphatic vasculature, acting as baroreceptors in the lymphatic and vascular systems, and sensing frictional force to determine vascular structure.3 Pathogenic variants in PIEZO1 have therefore been phenotypically associated with lymphatic malformation, perinatal edema, and nonimmune fetal hydrops, as well as hemolytic anemia and pseudohyperkalemia.2, 4 PIEZO1-related disorders that are due to biallelic loss-of-function variants include autosomal recessive lymphatic malformation 6 (LMPHM6, OMIM 616843) and disorders due to monoallelic gain-of-function variants include autosomal dominant dehydrated hereditary stomatocytosis (OMIM 194380).5 Despite significant phenotypic heterogeneity, both disorders may present with perinatal edema and nonimmune hydrops fetalis.5
Congenital lymphatic dysplasia is the hallmark feature of LMPHM6, resulting in severe impairment of vascular development, and commonly causing in utero demise. The associated primary lymphedema affects all body segments, causing pleural effusions, pulmonary and intestinal lymphangiectasia, facial and neck swelling, chylothoraces, and pericardial effusion. In some cases, hydrops fetalis has resolved postnatally with a concomitant recurrence of peripheral edema during childhood, typically in the lower limbs. Most reported cases of LMPHM6 have resultant pleural effusions that become chylous with the introduction of enteral feeds.5
More recently, PIEZO1 has been implicated as a potential mechanotransducer in osteoblastic bone formation. Osteoblasts respond to mechanical loading; thus, bone growth and maintenance is known to be impaired in microgravity environments and nonweight bearing patients. However, the mechanism by which this occurs has previously been uncertain.6 Recent research found that knockout mice with PIEZO1-deficient osteoblasts sustain spontaneous fractures in the setting of impaired bone density and strength. Patients with osteoporosis have been found to have reduced expression of the PIEZO1 protein.6 Likewise, simulated microgravity environments result in decreased osteoblastic function via suppression of PIEZO1 activity.7 Here, we present a patient with LMPH6 due to likely pathogenic homozygous loss-of-function PIEZO1 variants who has unexplained bone disease, the first reported case of significant bone disease in this phenotype.
2 CASE PRESENTATION
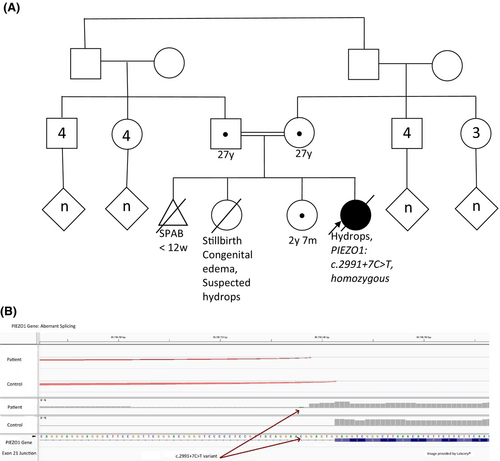
A female infant was born to a 28-year-old, gravida 4 now para 3012 Pashtun mother at an estimated 39 weeks' gestation. The pregnancy was notable for parental consanguinity but was otherwise uncomplicated. There was consistent prenatal care throughout with normal ultrasound scans and physical examinations reported. Medical records and detailed history from this pregnancy and the mother's prior pregnancies were unavailable. However, a previous live-born full-term sibling of the patient was reportedly “swollen at birth” and died of a “fluid issue” shortly thereafter. The patient has one living older female full sibling with normal growth, health, and development to date (Figure 1A).
The infant was delivered through thick meconium-stained fluids and was hydropic and hypotonic. Diuretic refractory edema worsened, with severe pitting edema and pleural effusions. After initiation of enteral formula and breast milk feeds on day 4 after birth, pleural effusions progressed, leading to further respiratory compromise necessitating high frequency ventilation by day 8 after birth. Enteral feeds were therefore discontinued; a thoracentesis was performed, and fluid analysis confirmed congenital chylothorax. Following the initial resuscitation, the patient experienced persistent hypotension, requiring maximal vasopressor support, and stress-dose steroids to maintain hemodynamic stability. At 18 days after birth, the infant underwent aeromedical evacuation to a center with a higher level of neonatal critical care services.
Following transfer, and for the remainder of her inpatient hospital admission, the infant experienced a complex clinical course primarily affected by severe peripheral and airway edema, chylothoraces, and bone disease.
3 METHODS
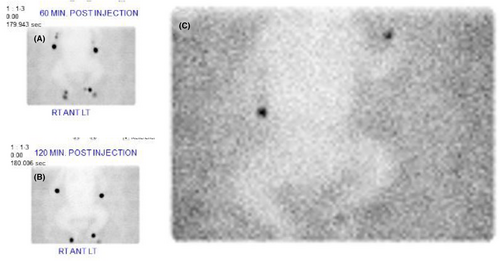
A Tc-99 m sulfur colloid lymphoscintigraphy study confirmed severe, diffuse, lymphatic dysplasia (Figure 2). An echocardiogram demonstrated normal cardiac function. An ophthalmologic exam was normal. The patient was found to have profound lymphopenia and nonhemolytic anemia. Lymphopenia was suspected to be complicated by chylothoraces and resolved following treatment of the chylothoraces with a medium chain triglyceride formula followed by prolonged nil per os status, octreotide infusion, and thoracostomy tube evacuation. Continued respiratory support was provided with high frequency ventilation to mitigate bilateral pleural effusions and pneumothoraces. Hypoalbuminemia resulted from persistent chylothoraces and was regularly treated with albumin infusions. At approximately 6 weeks of age, an extubation attempt proved unsuccessful due to persistent facial and airway edema. Tracheostomy was ultimately necessary, and the patient remained on intermittent mechanical ventilation.
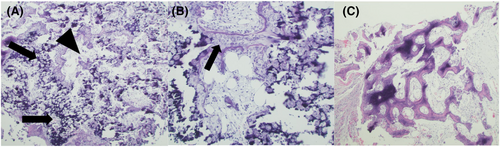
Later in the hospital course, atraumatic femur and humerus fractures were incidentally found on plain radiographs, as well as diffuse abnormal bone mineralization. Laboratory evaluation showed normal calcium, phosphorous, 25-hydroxy vitamin D, alkaline phosphatase, urine calcium, urine phosphate, and parathyroid hormone levels. There were no other known genetic causes of bone fragility. A bone biopsy was performed to evaluate for tumoral calcinosis, infectious processes, or other explanations for pathologic fractures (Figure 3). The biopsy revealed abnormal endochondral ossification, failure of trabecular maturation, and absence of zonal maturation of cartilage to bone.
Extensive genetic testing was performed, to include a karyotype and single nucleotide polymorphism array. The karyotype demonstrated a normal female chromosome complement (46, XX). The microarray was normal. Mitochondrial DNA testing and whole exome sequencing were performed through Medical Neurogenetics. Mitochondrial genome sequencing and deletion analysis as well as depletion studies were normal. Exome nuclear gene single nucleotide polymorphism sequencing detected a homozygous variant of uncertain significance in PIEZO1, c.2991 + 7C > T, near the donor splice site of exon 21. Follow-up RNA studies revealed altered splicing consistent with a novel splice junction caused by the c.2991 + 7C > T variant, extending the transcript 5 bases beyond the canonical splice site, predicted to result in frameshift and premature termination of PIEZO1 with loss of more than 1500 amino acids (Figure 1B). Exome sequencing also identified a homozygous variant of uncertain significance in LRP5 (Low Density Lipoprotein Receptor-Related Protein 5, OMIM 603506), c. 4049 T > G, p.Leu1350Arg. The patient's parents and 2-year-old sibling, with no history of bone fragility, were heterozygous for this variant.
4 CONCLUSION AND RESULTS
In our patient's case, the most pronounced feature of her disorder remains the generalized congenital lymphedema. Our patient's lymphedema was refractory to medical management. She remained tracheostomy dependent, despite overall healthy lung tissue, and succumbed to worsening pleural edema in the setting of the family's decision to provide palliative care. In most reported cases, if edema persisted, it was limited to a few body segments. Our patient; however, experienced persistent anasarca upon facility transfer at 4 months of age. Furthermore, our patient's lymphoscintigraphy showed no evidence of radiotracer movement after 24 h. These findings may suggest our patient's PIEZO1 variant leads to a more severe form of lymphatic malformation than has been previously reported. Our patient's PIEZO1 variant has not previously been reported in the literature. This case is also the first reported case of pathologic bone fractures in a patient with a loss-of-function PIEZO1 variant, implicating this variant as a novel genetic predisposition for fractures.
5 DISCUSSION
We described a patient with severe congenital lymphatic dysplasia that resulted from a novel variant in the PIEZO1 gene that caused aberrant splicing and resulted in premature termination of the protein. Due to this case, the PIEZO1, c.2991 + 7C > T variant was subsequently upgraded to likely pathogenic, and we concluded that this variant is responsible for our patient's phenotype. With respect to the homozygous variant found in LRP5, single pathogenic variants in LRP5 are associated with autosomal dominant osteopetrosis, osteosclerosis, and osteoporosis, while biallelic pathogenic variants are associated with autosomal recessive osteoporosis-pseudoglioma syndrome and exudative vitreoretinopathy. The observed variant frequency in South Asian control individuals in the gnomAD database is approximately 22-fold of the estimated maximal expected allele frequency for a pathogenic variant in LRP5, strongly suggesting the variant is a benign polymorphism found primarily in populations of South Asian origin. We do not think this variant plays any role in our patient's phenotype.
Significant research has identified PIEZO1 as an important mechanotransducer implicated in bone growth and maintenance. In a recent study, Wang et al. evaluated the role of PIEZO1 in the formation and remodeling of bone.8 The work postulated that osteoblasts use PIEZO1 to sense mechanical loading and promote bone resorption by osteoclasts. In PIEZO1 knockout mice, their study demonstrated that trabecular bone mass was significantly reduced compared to the wild type.8 Notably, they observed multiple bone fractures in weight-bearing appendicular bones postnatally. This supports the hypothesis that the PIEZO1 protein acts in the osteoblastic lineage cells to regulate bone remodeling by mechanical sensing. Furthermore, some studies have shown single nucleotide polymorphisms in PIEZO1 in patients who develop osteoporosis, suggesting another link to the importance of PIEZO1 ion channels on bone development and remodeling.8
Lab studies have shown that repeated small strains help to guide the modeling and remodeling phases of bone healing in patients who have undergone trauma. In contrast, in settings of complete nonweight bearing during the remodeling phase after a fracture, callus formation may be diminished, and healing can slow or fail all together. Excessive strain can result in a similar pattern of disordered formation.6, 8 While research has recently expanded significantly regarding the function of the PIEZO1 family of mechanoreceptors, there is still very little known about the role this protein plays in fetal development or the implications this may have on treatment for a variety of bone diseases.
PIEZO1 mechanotransduction is a critical process in bone growth and maintenance. Research demonstrates that the expression and function of the PIEZO1 protein is impacted by PIEZO1 variants both acutely, as detailed in this case presentation, and chronically, as it is under-expressed in individuals who develop osteoporosis.7 Importantly, the expression and function are also affected by the environment, as it is suppressed in microgravity situations.6 This case presentation, in concert with the recent literature on PIEZO1's role as a major skeletal mechanosensor impacting bone homeostasis, highlights the importance of PIEZO1 in multiple types of bone disease and offers PIEZO1 as a potential area of study for treatment and prevention of bone diseases.
AUTHOR CONTRIBUTIONS
Elizabeth H. Ketchum: Conceptualization; writing – original draft; writing – review and editing. Charles L. Groomes: Conceptualization; writing – original draft; writing – review and editing. Alexis N. Ghersi: Writing – original draft. Brian B. Graziose: Writing – original draft. Sharen C. Wilson: Writing – original draft. Sidney E. Zven: Writing – original draft. Rebecca L. Hicks: Data curation; resources; visualization. Michael A. Reott: Data curation; software; visualization. William A. Langley: Data curation; software; visualization. John P. Schacht: Supervision; writing – review and editing. Elizabeth V. Schulz: Conceptualization; supervision; writing – review and editing. Jerri Curtis: Conceptualization; supervision; writing – review and editing.
ACKNOWLEDGMENTS
Sofia C. Echelmeyer, Visual Information Specialist, Uniformed Services University, assisted with creating the graphical abstract.
6 FUNDING INFORMATION
This research did not receive any specific grant from funding agencies in the public, commercial, or non-for-profit sector.
CONFLICT OF INTEREST STATEMENT
Drs. Langley and Reott are employees of Labcorp.
ETHICS STATMENT
This article was approved for publication, given the signed parental and organizational consents, by the Public Affairs Office at Walter Reed National Military Medical Center (#9236, approved April 6th, 2023).
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
DISCLAIMERS
The views expressed in this article are those of the author(s) and do not necessarily reflect the official policy or position of the Department of the Navy, Department of the Army, Department of the Air Force, Department of Defense, or the United States Government. We are military service members (or employees of the US Government). This work was prepared as part of our official duties. Title 17, USC, §105 provides that “Copyright protection under this title is not available for any work of the US Government.” Title 17, USC, §101 defines a US Government work as a work prepared by a military service member or employee of the US Government as part of that person's official duties.
Open Research
DATA AVAILABILITY STATEMENT
Anonymized patient data will be available upon request.




