Clinical importance of distinguishing true anemia from dilutional pseudo-anemia: Consequences of a 3-year follow-up volume assessment in a heart failure patient
Key Clinical Message
In chronic heart failure, dilutional anemia and hypervolemia may occur due to plasma volume expansion, the latter sometimes exacerbated by an increase in red cell volume. Diagnosis and a therapeutic strategy require determination of vascular volumes.
1 INTRODUCTION
A major concomitant of chronic heart failure (CHF) is volume overload, affecting the interstitial space as well as the blood volume (BV), leading to both peripheral edema and intravascular congestion with concomitant organ damage.1, 2
The increase in BV is usually due to the expansion of plasma volume (PV), whereas red cell volume (RCV) may be decreased, constant, or increased.3, 4 In clinical practice, the BV parameters are not assessed routinely. Usually, weight change and the presence of edema are considered as indicators of volume status. RCV, and thus the total amount of hemoglobin available for oxygen transport, is also not measured directly, but are estimated from the hemoglobin concentration ([Hb]).
Anemia is common in patients with heart failure, with prevalence rates between 34%5 and 68%.6 The diagnosis of anemia is based on assessing the [Hb].7 After ruling out active bleeding, iron supplementation is often initiated, depending on the cause of the anemia.8 In some cases, usually in response to chronic kidney disease, erythropoiesis-stimulating agents (ESAs) are applied, whereas the routine use of erythropoietin has been associated with an increased mortality of CHF patients,9, 10 and therefore, has a class III recommendation in current heart failure guidelines.11
Noteworthy, as a consequence of an increased PV, about 30% of CHF patients diagnosed to be anemic are not featuring true anemia (lack of oxygen carriers), but so-called “dilutional anemia.”6, 12 Patients with dilutional anemia suffer from an excessive PV, “diluting” the RCV, and a significant proportion of patients with dilutional anemia might even have polycythemia.3, 4 However, the latter diagnosis necessitates a direct measurement of the red cell compartment.
Although some studies have measured BV parameters in heart failure patients, only few studies report on longitudinal data.13-15 Therefore, knowledge of the changes in the vascular parameters occurring in an individual patient throughout the course of real-world heart failure treatment is scarce. We here present an illustrative case, in which BV status has been determined from a purely observational perspective over a period of almost 3 years, in order to monitor the fluctuations that can occur in vascular parameters, in response to a real-world treatment throughout the progression of CHF in combination with chronic kidney disease.
2 CASE HISTORY
We report on a 78-year-old male patient, who first presented to the department of Sports Medicine/Sports Physiology of the University of Bayreuth/Germany in March 2006. While being treated for the cardiac conditions at the medical service in a regional center in Lower Saxony, Germany, the patient was followed up for assessment of BV, RCV, and PV, within a time period of 34 months, from a purely observational perspective. The medical history was established from the documentation available to us. The treatment was not influenced in any way by the findings derived from BV determination. The patient characteristics, when first presenting to the BV laboratory, are summarized in Table 1, and the timeline of BV measurements and medical events is presented in Table 2.
| Anthropometric data | Body mass 95 kg |
| Height 184 cm | |
| BMI 28.0 kg/m2 | |
| Hematological status | [Hb] 13.2 g/dL |
| Hematocrit 38.5% | |
| Cardiovascular diagnosis | Heart failure with preserved ejection fraction (HFpEF) |
| Nonobstructive coronary artery disease | |
| Severe tricuspid regurgitation | |
| Arterial hypertension | |
| Non-cardiovascular conditions | Low malignant B-cell lymphoma for 23 years |
| Status post choroidal melanoma with enucleation of the right eye 18 years ago | |
| Adenocarcinoma of the prostate with s/p curative prostatectomy 3 years ago | |
| Symptomatic gallstone disease 1 year ago | |
| G3 (KDIGO) chronic renal failure with nephrosclerosis (eGFR 31 mL/min/1.73 m2) | |
| Medication | Angiotensin II receptor antagonists (Lorzaar 50 mg) |
| Beta-blockers (Concor 2.5 mg) | |
| Furosemide (Lasix 40 mg) | |
| Aspirin (Ass 100) |
| Month 0 | First CO-rebreathing test to determine RCV, PV, and BV |
| Month 9 | As a consequence of low [Hb] (11.4 g/dL; iron 106 μg/dL, ferritin 71 ng/mL, transferrin 304 mg/dL), iron infusion (Venofer 20 mg; 1x/week) was initiated |
| Month 13 |
Progression of the coronary artery disease, and of tricuspid regurgitation, resulting in increasing right heart failure (left atrial diameter 60 mm, left ventricular end-diastolic diameter 54 mm, septum thickness 11 mm, mild mitral regurgitation and severe tricuspid regurgitation, right ventricular end-diastolic diameter 60 mm, left ventricular ejection fraction 50%) Two aorto-coronary bypasses (left-internal mammary artery on the left anterior descending artery, aortocoronary venous bypass on ramus marginalis), and, within the same surgery, a De Vega plasty of the tricuspid valve Administration of vitamin K antagonist (Marcumar) was initiated. Acute-on-chronic kidney failure and transient dialysis |
| Month 16 | Second CO-rebreathing test |
| Month 18 | [Hb] 9.3 g/dL, therefore transfusion of one unit of blood and first administration of darbepoetin alpha (20 μg /week) |
| Month 22 | Increase in darbepoetin alpha dosage to 40 μg /week |
| Month 28 | [Hb] 9.2 g/dL, increase in darbepoetin alpha dosage to 100 μg /week |
| Month 29 | Third CO-rebreathing test |
| Month 33 | [Hb] 14.7 g/dL, reduction in darbepoetin alpha dosage to 100 μg/3 weeks |
| Month 34 | Fourth CO-rebreathing test |
| Month 37 | Initiation of permanent dialysis |
| Month 41 | The patient was admitted to hospital with edema and dyspnea. The patient did not respond to treatment, and died of septic shock, due to infections caused by multidrug-resistant bacteria |
When the patient presented to our department, he had just been recompensed after acute cardiac decompensation. At that time, his diagnosis was heart failure with preserved ejection fraction, and he had a severe tricuspid regurgitation, as well as chronic kidney damage.
One year after the first presentation, and due to progression of the chronic coronary syndrome, the patient underwent two aorto-coronary bypasses (left-internal mammary artery on the left anterior descending artery, aortocoronary venous bypass on ramus marginalis) and, within the same surgery, a De Vega plasty of the tricuspid valve. Pulmonary hypertension, paroxysmal atrial fibrillation, and acute-on-chronic renal insufficiency occurred postoperatively, the latter initially leading to transient dialysis, and after 2 years, to chronic dialysis due to progression of his chronic renal disease.
From the first hospital admission, due to acute decompensation, the patient was treated continuously with furosemide. The body mass was stable at about 95 kg, until the bypass surgery, dropping to 78.5 kg after the surgery, and slightly increasing to 84 kg until the last observation of the BV parameters. Nevertheless, the occurrence of edema could never be fully prevented during the observational period.
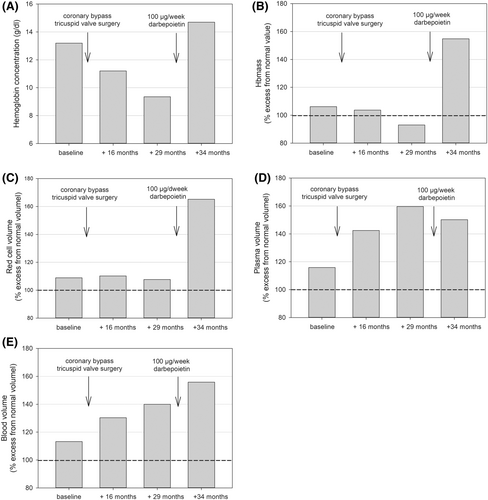
The [Hb] was in the lower normal range (13.2 g/dL) at first presentation, and remarkably oscillated during the following treatment period (Table 3; Figure 1A). It dropped to mild anemic values after 9 months ([Hb] 11.4 g/dL). Iron therapy was initiated by the treating nephrologist. Five months after the bypass surgery, [Hb] dropped to 9.3 g/dL (18th month). In addition to iron infusions, treatment with darbepoetin alfa (20 μg/week) was initiated. The dose was increased to 40 μg/week after 22 months and, as [Hb] remained at a low level (9.2 g/dL), to 100 μg/week after 28 months. Consequently, [Hb] increased within 5 months to 14.7 g/dL; the dosage was again reduced to 100 μg/3 weeks, resulting in decreased [Hb] to 10.3 g/dL after 36 months.
| Baseline | +16 months | +29 months | +34 months | |
|---|---|---|---|---|
| [Hb] (g/dL) | 13.2 | 11.2 | 9.3 | 14.7 |
| Hematocrit (%) | 39.9 | 35.1 | 31.9 | 46.4 |
| Hbmass (g) | 919 | 827 | 757 | 1272 |
| Hbmass (g/kg) | 9.7 | 10.3 | 9.1 | 15.3 |
| RCV (mL) | 2777 | 2592 | 2582 | 4002 |
| RCV (mL/kg) | 29.2 | 32.4 | 31.1 | 48.2 |
| PV (mL) | 4871 | 5525 | 6311 | 5995 |
| PV (mL/kg) | 51.3 | 69.1 | 76.0 | 72.2 |
| BV (mL) | 7648 | 8117 | 8893 | 9997 |
| BV (mL/kg) | 80.5 | 101.5 | 107.1 | 120.4 |
- Abbreviations: BV, blood volume; Hbmass, total hemoglobin mass in grams; PV, plasma volume; RCV, red cell volume.
3 METHODS
The vascular compartments were assessed by a CO-rebreathing method at four time points throughout the disease progression (see Table 2). The method is based on inhalation of a small volume of carbon monoxide, which increases COHb% by 4%–5%, which is inversely proportional to the total hemoglobin mass (Hbmass) and RCV with an accuracy of 1.5%.16, 17
4 RESULTS
At the time of initial presentation, Hbmass and RCV were within the normal range (where a deviation >8% from the optimal value indicates either an excess or deficit of volume1, 14); PV and BV were slightly elevated (16% and 13% of normal values, respectively; for absolute values, see Table 3). Hbmass (Figure 1B) and RCV (Figure 1C) remained in the normal range during the following months, while PV (Figure 1D) and BV (Figure 1E) increased to 160% and 140%, respectively, as a possible consequence of the combination of heart and kidney failure, resulting in a dilutional anemia. Five months after high dosing of darbepoetin, RCV had increased to 165%, whereas PV also remained elevated, leading to a further increase in the total BV to 156% (Figure 1C–E).
5 DISCUSSION
Some interventional studies have already described the effects of a single therapeutic intervention, for example, EPO administration,18 or implantation of a left ventricular assist device on vascular parameters.15 To our knowledge, however, this is the first report of vascular volumes repeatedly measured during the course of heart failure and anemia therapy over 3 years. The case highlights the difficulty of distinguishing between true anemia and dilutional anemia, and illustrates the extent of hypervolemia after ESA administration.
During the first months of treatment with iron substitution and low dosage with darbepoietin, the low [Hb] was the result of the PV increase, that is, there was hardly any true anemia, but rather a dilutional anemia, which is described in approximately 30% of CHF patients,12 and can be considered as typical for CHF patients with HFrEF.1 Between the third and fourth assessment of vascular volumes, the continuously elevated PV masked the ESA-induced polycythemia, so that a considerable hypervolemia developed, due to both massively increased plasma and erythrocyte volume. These insights concerning the volume status were only possible because of direct observation of the intravascular volumes.
Recently, Ahlgrim et al. described CHF patients with an expanded PV at significantly higher risk of mortality.14 They found a cutoff value of 1800 mL/m2 body surface area, the exceeding of which increased the mortality risk independently of RCV. In the case presented here, PV exceeded this cutoff value by 70% (3080 mL/m2) at the third measurement after 29 months, and by 60% (2900 mL/m2) after 34 months. The importance of such knowledge is demonstrated in a pilot study, where determination of blood and PVs in decompensated heart failure, and subsequent therapeutic normalization of the volume status, is reported to be associated with remarkably lower mortality of 30 and 365 days, when compared with propensity-matched controls.19
After 28 months, due to the kidney disease and the accompanying low [Hb] (9.2 g/dL), the darbepoetin dose was increased to 100 μg/week for the following 5 months, resulting in a 68% increase in total Hb mass and thus an increase in [Hb] to 14.7 g/dL. Today, however, the application of ESAs in anemic patients to achieve normal [Hb] (>13 g/dL) is not encouraged,11 as the cardiovascular risks significantly exceed the benefits10, 20 in this group of patients. In patients suffering from chronic kidney disease and diabetes, darbepoetin alfa treatment (with a target [Hb] of 13 g/dL) also did not reduce the risk of either of the two primary composite outcomes (either death or a cardiovascular event or death or a renal event), and was associated with an increased risk of stroke.21 The reasons for this are believed to include higher blood viscosity, thromboembolic events, and hypertension.2 Although we cannot give causal reasons for the malignant course in the present case, the undifferentiated treatment of true and dilutional anemia is likely to play a decisive role. Due to the seemingly beneficial effects of EPO treatment, in the form of normalization of [Hb], in addition to the already remarkably high PV, RCV was elevated to 165% of normal in our patient, resulting in volume overload to 156% of normal, which in the present case may have aggravated the course of heart failure.
6 CLINICAL IMPLICATIONS
Measurement of peripheral Hb concentration alone may contribute to a misperception of the patient's true volume and anemia status, which has implications not only for patient outcomes but also for management strategies.
For example, Strobeck et al.19 reported 30-day rates of readmission, and 365-day mortality to be lower (12.2% vs. 27.7% and 4.9% vs. 35.5%) in a group of heart failure patients with a volume-guided therapy, in comparison with a propensity-matched control group, although the length of stay was longer (7.3 vs. 5.6 days).
This kind of therapy requires an initial determination of the volume status and anemia status either by radioactive methods19 or by the CO rebreathing technique,22-24 and a longitudinal control of the hematocrit value. With this knowledge, dilutional anemia can be selectively treated by diuretic measures, and true anemia by ESAs, and, if necessary, also by diuretics. Ahlgrim et al.14 and Strobeck et al.19 have proposed treatment targets in this context.
As indicated throughout this paper, a definite diagnosis of dilutional anemia appears hardly feasible without direct assessment of the intravascular volumes. Such an assessment seems necessary to directly guide therapy. Noteworthy, in recent years, SGLT-2 inhibitors have entered clinical practice as a class of compounds that reduce mortality across the entire range of left ventricular ejection fraction, and appear to favorably influence dilutional anemia, by reducing PV while slightly increasing red blood cell mass. The same might hold true for another therapeutic approach: Intravenous iron therapy of heart failure patients with iron deficit has been shown to reduce readmission rates.8 A recent study, albeit assessing the PV using estimation methods, has described an unexpected, but favorable effect of iron substitution on dilutional anemia. In their work, they describe a reduction in PV and body weight, following iron administration.25 From a clinical and hemodynamic perspective, the relevance of PV for the venous systems cannot be underestimated; the important role of the venous system, and the concepts of stressed BV and unstressed BV with their direct effect on cardiac loading conditions, have, once more, recently been highlighted.26
7 CONCLUSIONS
This case illustrates the development of dilutional anemia due to increased PV, and furthermore the development of severe hypervolemia, when, additionally to expended PV, RCV is elevated as a result of treatment with ESAs. None of these complications is reflected by assessing only [Hb] or hematocrit. Therefore, routine determination of vascular volumes, which is readily feasible with CO-rebreathing methods, could lead to more individualized treatment of both true anemia and dilutional anemia, and could help to avoid volume overload.19 In this context, other workgroups have also proposed determination of BV to monitor ESA response in CHF patients.18
AUTHOR CONTRIBUTIONS
Walter F. J. Schmidt: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; resources; validation; visualization; writing – original draft; writing – review and editing. Christoph Ahlgrim: Conceptualization; data curation; formal analysis; supervision; validation; writing – original draft; writing – review and editing.
ACKNOWLEDGMENT
Open Access funding enabled and organized by Projekt DEAL.
FUNDING INFORMATION
The publication was made possible by the open access fund of the University of Bayreuth and the German Research Foundation (DFG).
CONSENT
Written informed consent was obtained from the patient's daughter to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




