Acute patellar dislocation during isokinetic knee strength testing: A case report
Abstract
Key Clinical Message
Isokinetic testing is a maximal muscle strength test which requires adequate patient's preparation and observer's care. While the available data suggests that isokinetic devices are safe, their use may rarely cause severe injuries. The screening of predisposing anatomical factors could help preventing injuries before testing.
A 29-year-old athletic man presented an acute patellar dislocation on a healthy right knee during isokinetic muscle strength testing, which was conducted in the setting of an intensive physical rehabilitation program, for persistent left knee pain after arthroscopic surgery for meniscal tear. This is the first case to occur in an adult male without clear risk factors such as patellar dysplasia. Predisposing factors may include slightly elevated patellar tilt and lateral shift compared to the contralateral knee (researched from subsequent review of pre-injury X-rays), and an elevated quadricipital strength in the context of recreational bodybuilding. The dislocation occurred during eccentric extension phase of testing. Medial patellofemoral ligament reconstruction was conducted 6 months later. Isokinetic muscle strength testing is generally considered as a safe method, despite limited data on the devices' safety. Since severe injuries might rarely occur, adequate patient preparation is needed, as well as the screening of predisposing factors.
1 INTRODUCTION
Isokinetic devices are widely used in musculoskeletal rehabilitation and sports medicine to assess muscle strength, especially in knee testing after trauma or surgery.1 Developed in the late ‘60s, they became popular in the ‘80s and are since considered the gold standard of muscle strength assessment.1 Their main advantage is in providing information on dynamic muscular contractions like no other clinical method of measurement.2 Generally considered safe,2 we discuss the safety issues surrounding these devices with the support of data from the literature. While rare, injuries can happen such as patellar dislocation, which is a major injury that may have long-term consequences such as instability of patellar origin, pain, recurrent dislocation and patellofemoral osteoarthritis.3 We report the case of an acute patellar dislocation of the patient's healthy knee during eccentric testing on an isokinetic device, which was conducted to define rehabilitation objectives for the contralateral operated knee, and which is the first case to occur in an adult male without clear risk factors such as patellar dysplasia.
2 CASE PRESENTATION
2.1 Patient information
A 29-year-old Caucasian male, recreational bodybuilder, with no prior history of patellar dislocation, trochlear dysplasia, patellar instability symptoms or hyperlaxity, was treated in our rehabilitation department for persistent exertional left knee pain 8 months after arthroscopic meniscus suture for a traumatic lateral meniscus tear. Early rehabilitation was delayed by a postoperative DVT in the left lower leg successfully treated with anticoagulants. Due to the high physical demands of the work sought by the patient (personal trainer), an intensive rehabilitation program was deemed necessary.
2.2 Timeline
To assess knee strength and further define rehabilitation goals, an isokinetic test was performed during the second week of the program. Isokinetic strength testing is a computerized measurement of muscle function and is the gold standard for muscle strength assessment.1 With dynamometers, it evaluates maximal joint torque production at a specified velocity of limb movement. At this point, the patient was fit to be evaluated, with the aim of assessing flexion and extension strength of both knees using concentric and eccentric modes of contraction at different angular velocities. He was unfamiliar with isokinetic testing but had extensive experience with open-chain exercises from his bodybuilding background. Instructions on the test were given to him by an experienced sports physical therapist and then a warm-up was performed (cycling followed by isokinetic warm-up). The concentric mode was first performed on the healthy non-operated right knee at 240°/s and 60°/s with no problems reported, the patient being asked to give regular feedback during the test. The eccentric mode was then initiated where the patient experienced sudden knee pain (caused by an acute patellar dislocation as later confirmed by MRI) during the eccentric contraction cycle, on the third repetition. The knee flexion angle noted was about 20° during the eccentric component of quadriceps testing. The angular velocity was set at 60°/s, but the curves could not be recorded because of the machine disengagement.
2.3 Clinical findings
A reflex syncope with a loss of consciousness followed the injury for about 1 min.
Due to immediate spontaneous reduction, the dislocation could not be clearly seen by the examiner. Diffuse right knee pain and intra-articular effusion were noted.
2.4 Diagnostic assessment
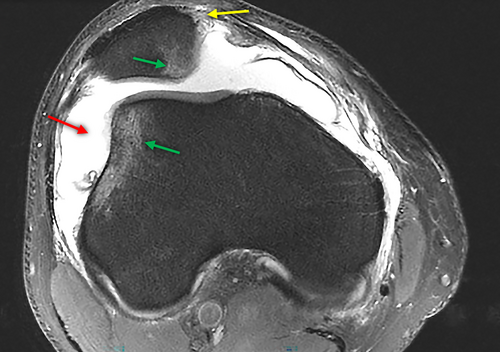
An MRI was performed the following day to evaluate possible intra-articular damage and to confirm the diagnosis of patellar dislocation, to further assess the severity of structural damage, and to better guide the treatment decision making. It showed typical lesions following patellar dislocation: bone bruising on the medial aspect of the patella and on the lateral femoral condyle, medial patellofemoral ligament (MPFL) tear and joint effusion/hemarthrosis (Figure 1). There was also post-traumatic grade II patellar chondropathy on the internal aspect. The anterior cruciate ligament was intact.
2.5 Therapeutic intervention
Immediate management included rest, elevation, compression, icing and painkillers. The patient was instructed to wear a knee extension split for 2 weeks while reducing weight bearing with crutches for a few days. The intensive rehab was discontinued, and the patient was discharged with a scheduled appointment with an orthopedic surgeon.
2.6 Follow-up and outcomes
An outpatient physiotherapy program was later resumed to include treatment of the recently injured right knee. MPFL reconstruction was conducted 6 months later. The patient reported satisfactory results in terms of stability 6 months after surgery, with residual pain during demanding physical activities.
3 DISCUSSION
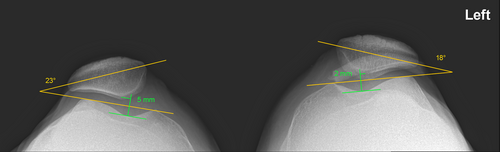
Our case is unusual in that a serious injury occurred on a healthy knee and on a testing device that is not generally known to cause serious injuries.1 This unexpected injury led to the search of anatomical predisposing factors for dislocation on available plain radiographs pre-injury. On the X-Rays, compared to the left side on the patellar axial view, there was a slightly elevated patellar tilt (23° vs.18° on the left) with a lateral shift of the patella on the right side (Figure 2), which could be a predisposing factor.4 On the contrary, there was no sign of patella alta, patella dysplasia or true trochlear dysplasia. No CT-scan was conducted before or after the injury to measure the tibial tuberosity to trochlear groove (TT-TG) distance, but the post-injury MRI did not show any signs of such a problem. Also, while trochlear depth and sulcus angle were inside the normal limit on the X-rays, the MRI showed a flattened surface of the trochlear groove (see Figure 1).
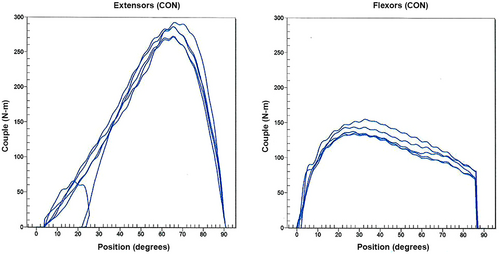
On the biomechanical perspective, it is worth mentioning the patient's general elevated strength given his history of bodybuilding training, weighing 102 kg at the time of testing. Just before the traumatic event, the peak torque of the right quadriceps was measured at 293 N-m on the concentric testing (Figure 3), the mean value averaging 170–200 N-m in the general population.5 This could be a contributing factor for an increased lateral vector force on the patella leading to dislocation. While the precise Q angle was not recorded, there was no excessive knee valgus clinically. Patient's installation seemed correct on a CSMi device in good conditions (showed in Figure 4), with no clear evidence of increased tibial external rotation as specified by the physical therapist, experienced in isokinetic testing and in sports physical therapy.

Three other cases of accidental patellofemoral dislocation on an isokinetic device are reported in the literature, and they significantly differ from our case. The first case reported by Maletius in 1994 is the only other case of primary dislocation on a healthy knee, occurring in a 24-year-old woman later known to have trochlear dysplasia.6 Major differences with our case included sex, the presence of an important structural risk factor, angular velocity (180°/s vs. 60°/s in our case) and knee flexion angle at the time of dislocation (70° vs. 20° in our case). Peak torque just before dislocation was recorded at about 210 Nm, which is significantly lower than in our case (293 Nm). The other two reported cases were redislocations, which occurred in a study led by Moström on the long-term follow-up of nonoperatively and operatively treated acute primary patellar dislocation in skeletally immature patients, a very different context compared to our case. The dislocations also occurred during the eccentric mode of contraction, while no other relevant details were given (age, sex, risk factors, angular velocity, or knee flexion angle).7
In rehab centers, most patients already have plain radiographs or other imaging prior to isokinetic muscle testing. It seems valuable to search for predisposing factors on the existing imaging, especially trochlear dysplasia, to better appreciate the risks for injury during isokinetic testing. This is also especially true in athletes given the higher forces generated on joints and tendons. The added value of comparative study should also be considered when prescribing plain X-rays.
This injury also led us to question the general safety of isokinetic devices. We conducted an overview of the literature using Pubmed, CINHAL and Cochrane databases with the following keywords: isokinetic, injury, safe, safety, dynamometer, muscle testing, device, and training. Major devices' brand names were also used (Biodex, Cybex, Kincom, Kintrex, Lido, Merac, Myostar, Myotrax). This strategy yielded only two published case reports of primary injuries on an isokinetic device. The first one was the other case of patellar dislocation in a 24-year-old woman with trochlear dysplasia,6 already mentioned above. The other one was a hamstring-muscle strain in a 25-year-old professional rugby player.8 Numerous papers mentioned the undesirable effects of isokinetic eccentric contractions known as DOMS (Delayed Onset Muscle Soreness).9-13 One study conducted on 230 participants reported 2 cases of acute severe back pain and one case of syncope during trunk testing, all requiring transportation to the emergency room.14 Another study also reported two cases of patellar redislocations on eccentric knee testing,7 as mentioned above (follow-up of acute patellar dislocation treated operatively or conservatively on skeletally immature patients).
It is worth noting here that every reported case of patellar dislocation during isokinetic testing happened during the eccentric mode of contraction. This is somehow unsurprising since it's the mode that generates the highest force in a given muscle.15
Most reviews and articles on isokinetic testing focus on muscle physiology and on its reliability and validity in rehabilitative or training protocols. Relevant references cited in selected articles were also considered, regardless of the year of publication, with no further specific findings on isokinetic devices' safety. One report mentions the lack of evidence for devices' safety.16 Moreover, isokinetic testing is generally considered as a safe method,2, 17, 18 one common argument being that the resistance will never exceed the force applied by the subject.16, 19 Furthermore, it is worth mentioning here that isokinetic devices must be FDA approved and/or CE certified.
Experienced physical therapists specialized in isokinetic testing were consulted for relevant literature references. They pointed to one larger review focusing on the use and interest of isokinetic devices in muscle evaluation and rehab, conducted by a working group of the then French National Agency for Health Evaluation and Accreditation (ANAES, now HAS).1 It referenced one article that was evaluating the risk of injury on isokinetic devices and their preventive measures.20 In that paper, the authors reported 6 injuries in 1000 patients (equivalent to roughly 3500 sessions over 3 years), only one of which was severe (patellar tendon rupture, the others being acute patellofemoral pain syndrome and hamstring strains). They also made an inquiry in 40 different centers (over 7 different countries): on the 17 answers received, 30 adverse events of variable severity were reported (with no specification of their incidence). It included scapulohumeral dislocation, muscular elongation of the triceps brachii, exacerbation of scapulohumeral pain, meniscal lesion, patellar lesion (pain, subluxation, patellofemoral syndrome), and haematoma caused by the fixation strap of the isokinetic lever.
We can summarize four different types of risks and adverse effects of isokinetic testing, as shown in Table 1 below.
| Machine-related | Pain and hematoma on fixation strap.1 |
| Joint-related | Dislocations and subluxations (mostly shoulder and patella), patellofemoral pain syndrome.1, 6, 20 |
| Musculo-tendinous | Mostly DOMS (especially on the eccentric component of testing), strains, tendon rupture.1, 9-13, 20 |
| Cardiovascular | Rise in heart rate and/or arterial pressure, ECG changes, reflex syncope.1, 14, 21 |
Other factors contributing to a decreased risk of injury include patient installation following strict protocol, machine robust settings, automatic machine resistance adjustment to patient's force, no initial inertia to overcome, constant angular speed, machine automatic disengagement in case of an emergency.
Factors contributing to an increased risk of injury include higher load on joint (isolated joint exercise),22 open-chain type of movement, maximal muscle strength type of testing.
This paper, although providing a detailed overview of safety issues regarding isokinetic testing, has limitations. As a case report, we are basing ourselves on a singular observation from which it is not possible to make solid generalizations. Another limitation is that the machines and their settings may vary, as may the test protocols.
In conclusion, despite limited available data concerning isokinetic devices' safety, we can probably assume that isokinetic testing is generally safe even if severe adverse events may rarely occur. To minimize risks, users should be mindful of technical-related factors, such as adequate subject installation, and patient-related factors, such as proper warm-up and research of predisposing risks for injury.
4 CONCLUSION
We report only the second published case of primary patellar dislocation caused by isokinetic testing in a 29-year-old male, the first without major intrinsic predisposing factors such as trochlear dysplasia or patella alta. While the available data suggests that isokinetic devices are safe for muscle testing and training, their use may rarely cause severe injuries.
Isokinetic testing is a maximal muscle strength test which requires adequate patient's preparation and observer's care, especially in the case of athletes with elevated muscular strength such as in the present case. The screening of predisposing anatomical factors could help preventing injuries before isokinetic testing: indications of trochlear dysplasia, patella alta, or elevated patellar tilt or shift on available imaging; evidence of elevated muscular strength, Q angle or tibial external rotation.
AUTHOR CONTRIBUTIONS
Obrenovic Mihailo: Investigation; visualization; writing – original draft. Luthi François: Supervision; writing – review and editing.
ACKNOWLEDGMENTS
None.
FUNDING INFORMATION
None declared.
CONFLICT OF INTEREST STATEMENT
Authors state no conflict of interest.
ETHICS STATEMENT
The conducted research is not related to either human or animals use. Although a human patient is involved, this is a case report for which no ethics committee consent is required.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.




