Hereditary epidermolytic palmoplantar keratosis due to a novel desmoglein-1 mutation: A case report
Kevin Koschitzki and Bernadett Kurz contributed equally to this study.
Abstract
Key Clinical Message
Keratosis palmoplantaris striata type I (SPPK-I) is a rare autosomal-dominant type of hereditary epidermolytic palmoplantar keratoderma, which can be caused by mutations in desmoglein-1 (DSG-1). Patients suffer from hyperkeratotic plaques and painful palmoplantar fissures. Unfortunately, treatment options including salicylic vaseline, topical corticosteroids, phototherapy, and retinoids are inefficient.
Hereditary palmoplantar keratodermas (PPKs) represent a heterogeneous group of rare skin disorders with epidermal palmoplantar hyperkeratosis. Mutations in the desmoglein 1 gene (DSG1), a transmembrane glycoprotein, have been reported primarily in striate PPKs. We report a patient with keratosis palmoplantaris striata type I (SPPK-I) with a specific pathogenic variant [c.349C>T, p.(Arg117*)] in DSG1. Despite increased understanding, effective treatment options for PPK, including SPPK-I, remain limited.
1 INTRODUCTION
Hereditary palmoplantar keratodermas (PPKs) are classified either by their genetic mutation or by their clinical picture. The importance of identifying isolated forms from syndromic entities has major clinical and therapeutic relevance.1 More than 69 different forms of PPK are known and can involve mutations in keratin (KRT), which are generally the most common subtypes and cause more diffuse forms often linked to epidermolytic ichthyosis.2 PPK can also be divided into four different types according to the clinical appearance of the hyperkeratosis: striate, punctate, focal, and diffuse PPK. Diffuse PPKs are the most common and striate PPK appears to be rare, and are commonly associated with syndromes, such as woolly hair syndrome.3 Mutations in DSG1 cause striate SPPK-1; striate SPPK-2 is caused by desmoplakin (DSP) mutations and SPPK-3 results from heterozygous mutations in KRT1.4
We report a case of keratosis palmoplantaris striata type I (SPPK-I) with a novel specific pathogenic variant [c.349C>T, p.(Arg117*)] in DSG1, highlighting the genetic basis of SPPK-I and its association with altered desmoglein function.
2 CASE HISTORY
2.1 Patient information
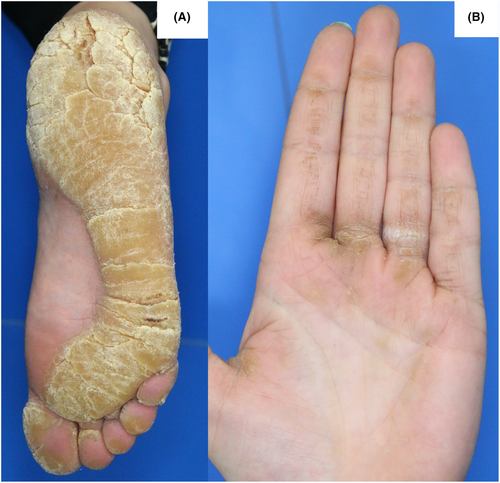
A 31-year-old woman presented with painful palmoplantar hyperkeratotic plaques and rhagades (Figure 1A,B) that first appeared during puberty. She did not suffer from skin fragility, blistering, hair or nail involvement. There was no history of cardiac or any other internal diseases. Two of her six siblings as well as her father had the same skin condition. History of consanguinity in parents may point toward the presence of recessive variants. In our case, the parents were not consanguine. None of the family members had additional symptoms (e.g., deafness, cardiological involvement, and mental retardation).
2.2 Clinical findings
Upon physical examination, striated yellowish to brownish, spongy hyperkeratoses were seen on the palmoplantar side in pressure-exposed areas. In the plantar region, deep rhagades up to 2 cm long were also seen in the area of the hyperkeratoses.
3 METHODS
3.1 Diagnostic assessment
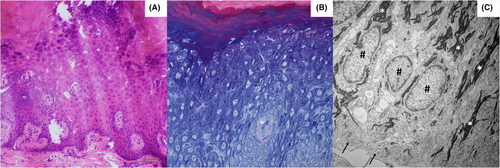
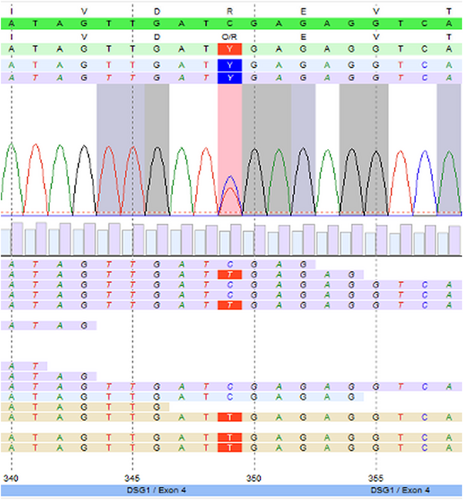
The patient underwent a lesion biopsy and histopathology of the ridged skin of the palm revealed orthohyperkeratosis and irregular acanthosis with a prominent stratum granulosum as well as akantholysis (Figure 2A,B). Transmission electron microscopy showed markedly elongated basal keratinocytes as well as numerous tonofilaments (Figure 2C). Next generation sequencing (NGS; Illumina MiSeq, 2 × 150 bp, paired end) revealed a novel heterozygous pathogenic variant in exon 4 of desmoglein-1 (DSG1) [c.349C>T, p.(Arg117*)] (Figure 3) concluding the diagnosis of SPPK-I. This variant causes a premature stop and is assumed to result in loss of function (haploinsufficiency) and was classified as 5 (pathogenic) according to the American College of Medical Genetics and Genomics classification. No pathogenic mutations in AQP5, CARD14, CAST, CTSC, GJB2, JUP, KRT1, KRT9, MBTPS2, NLRP1, POMP, SERPINB7, SERPINB8, SLURP1 or TRPV3 were observed.
3.2 Therapeutic interventions and outcome
Treatment initially consisted of topical 20% salicylic-vaseline ointments and 1 mg/g mometasone furoate applied on a once-daily basis. After 6 weeks, there was no improvement. Psoralen plus ultraviolet A therapy consisting of 45 sessions in a time span of 4 months resulted in a moderate but overall insufficient amelioration. Due to persisting hyperkeratosis with only slight improvement in inflammation, topical corticosteroid therapy was discontinued in favor of systemic acitretin 20 mg once daily. However, the skin condition did not improve.
4 DISCUSSION
In summary, our female patient presented with painful palmoplantar fissures and hyperkeratotic plaques. Histopathology revealed orthohyperkeratosis as well as an irregular acanthosis with a prominent stratum granulosum as well as acantholysis. NGS showed a heterozygous pathogenic variant in exon 4 of DSG1 [c.349C>T, p.(Arg117*)]. These findings could indicate a type of palmoplantar keratosis. Hereditary PPKs can be classified either by their genetic mutation or by their clinical picture.2 The importance of identifying isolated forms from syndromic entities has major clinical and therapeutic relevance.
The clinical history is crucial to differentiate between acquired and congenital causes of palmoplantar hyperkeratosis. Acquired causes would include atopic dermatitis, psoriasis, HIV infection, or exposure to chemicals such as arsenic and many more.5 None of these were present in our patient, so we considered whether there might be a genetic cause, which leads to NGS. More than 69 different forms of PPK are known and can involve mutations in KRT, which are generally the most common subtypes and cause more diffuse forms often linked to epidermolytic ichthyosis.2, 6 Other forms are due to variants in loricrin (which causes mutilating PPK), connexins (often related to syndromal PPKs), as well as variants in cathepsin, aquaporin, and chloride channels.4, 5 In our case, we found a novel heterozygous pathogenic variant in exon 4 of DSG1 [c.349C>T, p.(Arg117*)], which fits the clinical diagnosis of keratosis palmoplantaris striata type I (SPPK-I).
While the name keratosis palmoplantaris striata and areata type I already indicates the clinical subgroup of the rare “striate” forms which are separated from diffuse, focal, and punctuate types, the disease is caused by a mutation of DSG1 and therefore belongs to the genetic subgroup of desmosomopathies.
Mutations in DSG1 cause striate SPPK-1 (characterized by localized lesions in the majority of cases), Striate SPPK-2 is caused by DSP mutations (characterized by more focal lesions with fissures), and SPPK-3 results from heterozygous mutations in KRT1 (diffuse plantar changes).4
DSG-1 is mainly expressed in the suprabasal layers of the epidermis and mucosa, and codes for the protein desmoglein. It consists of the Greek words “desmos” and “glein” which stand for “tie” and “glue like,” respectively. Desmoglein creates calcium-dependent heterophilic bindings with desmocollins of adjacent cells in its extracellular domain, while the intracytoplasmic domain binds plakoglobin and links keratin cytoskeletons.7 DSG-1 is therefore essential for the intercellular adhesive function of desmosomes which are in general abundant in cells prone to higher amounts of mechanical stress.7-9 In SPPK-1, the mutation of DSG1 causes an alteration in the extracellular domain of desmoglein,7 which leads to haploinsufficiency via nonsense-mediated mRNA decay. In severe skin dermatitis, multiple allergies and the metabolic wasting (SAM) syndrome, there is a significant reduction or absence of DSG1 from the cell membrane. This deficiency may arise from various factors such as failure in DSG1 localization, decreased DSG1 expression, or nonsense-mediated mRNA decay.10 DSG1 also interacts with Erbin, thereby inhibiting the Ras/MAPK pathway, which leads to elevated Ras activity, resulting from inadequate or absent DSG1.11
Diagnosis is based on the clinical findings, histology and positive family history given the autosomal dominant inheritance. Despite the clinical, histological, and electron microscopic features of the disease, genetic testing is required for exact diagnosis.3
Furthermore, syndromic PPK must be ruled out. Potential symptoms pointing toward syndromic PPK include deafness, cardiologic involvement, or mental retardation.2
Treatment options consist of keratolysis and mechanical relief by wearing customized shoes to reduce pressure.4 Systemic retinoids have to be used carefully in epidermolytic PPKs, which can exacerbate upon medication with acitretin or alitretinoin.
Unfortunately, treatment options of PPKs are still not promising despite the wide knowledge of the individual forms and their pathophysiology.
This case presents SPPK-I, a rare hereditary skin disorder, resulting from mutations in the DSG-1 gene, causing painful palmoplantar fissures and hyperkeratotic plaques. A specific novel pathogenic variant [c.349C>T, p.(Arg117*)] in DSG1 was identified, highlighting the genetic basis of SPPK-I and its association with altered desmoglein function. The mutation leads to characteristic longitudinal hyperkeratosis on palms and soles. SPPK-I is part of a diverse group of over 69 palmoplantar keratosis (PPK) forms, diagnosed based on clinical findings and family history. Despite increased understanding, effective treatment options for PPK, including SPPK-I, remain limited.
AUTHOR CONTRIBUTIONS
Kevin Koschitzki: Investigation; writing – original draft. Bernadett Kurz: Writing – review and editing. Julia Schreml: Formal analysis. Judith Fischer: Conceptualization; writing – review and editing. Alrun Hotz: Formal analysis. Christoph M. Hammers: Writing – review and editing. Mark Berneburg: Writing – review and editing. Dennis Niebel: Formal analysis; writing – review and editing. Stephan Schreml: Conceptualization; formal analysis; resources; writing – original draft; writing – review and editing.
ACKNOWLEDGMENTS
The authors have nothing to report. Open Access funding enabled and organized by Projekt DEAL.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
All Authors have no conflict of interest to declare.
ETHICS STATEMENT
Ethical approval is not required for this study in accordance with local or national guidelines.
CONSENT
Written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available upon reasonable request.




