Extralobar pulmonary sequestration: A case report and literature review
Key Clinical Message
Pulmonary sequestration is a congenital malformation of lung development in which part of the lung tissue is separated from the normal lung during the embryonic phase and develops separately and receives blood supply from an aberrant systemic artery forming a nonrespiratory mass. In brief, early in embryonic development, certain tissues that should have atrophied and been gradually absorbed are left behind due to impairment of the atrophy process and form anomalous branches of the aorta, which pull parts of the lung tissue, isolating them from normal lung tissue and bronchi, and thus forming separate lung tissue. According to the relationship of the mass to the pleural covering, pulmonary sequestration can be divided into two types, intralobar pulmonary sequestration (ILS) and extralobar pulmonary sequestration (ELS), of which approximately 75% of cases are ILS, but ELS is less common. Symptoms are not obvious in either type, making diagnosis and differential diagnosis more difficult. Here we report a 33-year-old patient with only insignificant abdominal distension who was eventually diagnosed with retroperitoneal ELS.
1 INTRODUCTION
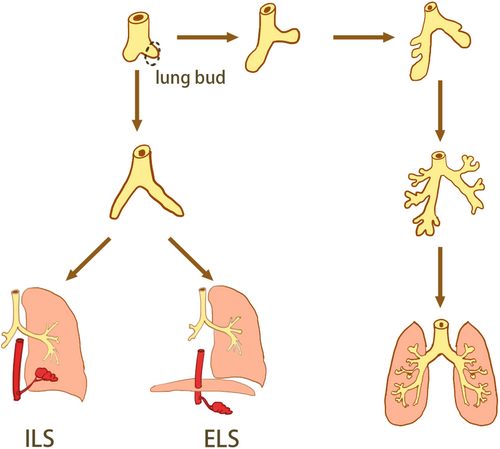
The pathogenesis of pulmonary sequestration (PS) is unclear and is currently considered to be common with the parapneumonic bud theory, the traction theory, and vascular development theory, but the parapneumonic bud theory is currently recognized, which anatomically classifies pulmonary sequestration into two types (Figure 1), intralobar pulmonary sequestration (ILS) and extralobar pulmonary sequestration (ELS), based on the relationship between the mass and the pleural covering.1 PS arises from the primitive foregut, if the pulmonary bud develops before the pleura and is covered by adjacent lung tissue, ILS is present and if this pulmonary bud develops later than the pleural development and grows independently, it results in ELS.2
The incidence of PS in congenital malformation of the lung is 1.1%–1.8%.1 ELS can occur in the thoracic cavity, mediastinum, pericardium, and intra- or sub-diaphragm,3 and the retroperitoneal ELS of the 33-year-old man mentioned in this case is even rarer, with only 10%–15% of ELS being found in retroperitoneal sites.4
2 CASE REPORT
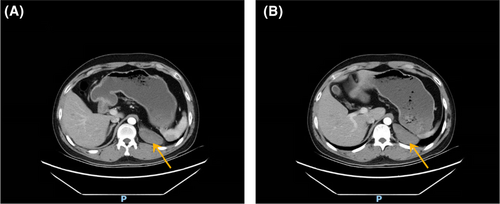
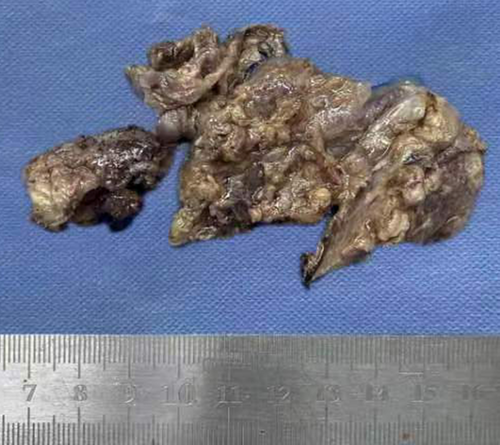
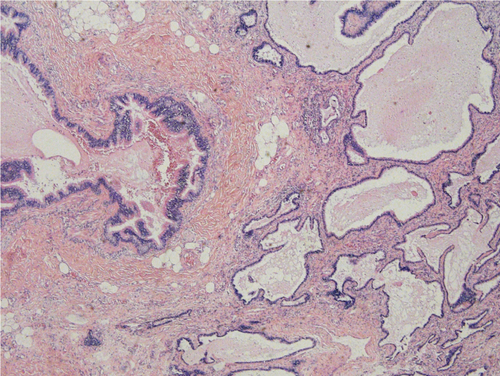
A 33-year-old man was admitted to our hospital for a retroperitoneal mass. He had been admitted to a local hospital for abdominal distension and computed tomography (CT) of the abdomen revealed an space-occupying lesion in the left diaphragm. The patient only felt abdominal distension and had no other significant abnormalities in his physical examination, medical history, or family history. The patient reported that he was prone to upper respiratory tract infections when he was young and had been misdiagnosed with “mycoplasma pneumonia.” He also felt chest tightness and dyspnea after exertion, and the left side of his face and left ear were easily flushed, often with acid reflux and heartburn. However, these symptoms disappeared in adulthood. Previously, a malignant tumor of the abdominal cavity, retroperitoneal schwannoma was suspected. The patient's vital signs were stable and laboratory tests showed aldosterone 60.21 pg/mL, and the rest of the indicators were within normal limits. CT of the upper abdomen and chest revealed a well-delineated, soft tissue, dense structure measuring 7.5 × 2.5 cm that was located in the left diaphragm and the suspected blood supply artery from the abdominal aorta could be identified on CT (Figure 2). Ultrasound-guided puncture biopsy showed little fibrous connective tissue and mucus, and the diagnosis was not clear before surgery. Laparoscopic exploration was performed, and the intraoperative mass was seen to be a multicystic mass, measuring 10 × 5 × 4 cm, containing black mucus, reaching posteriorly to the diaphragm and inferiorly to the superior margin of the pancreas, with an extensive base and a deep location. The upper edge of the mass was seen to be tightly adherent to the pleura, and its base was deeper and more heavily adherent to the diaphragm. Therefore, laparotomy was performed, and the mass was peeled away from the surrounding tissues and excised completely. Macroscopy revealed a multicystic mass with a gray–red color, and when the resection was completed, the mucus contained in the mass was drained (Figure 3). The pathological diagnosis was retroperitoneal ELS (Figure 4). The patient recovered uneventfully and was discharged on postoperative Day 8.
3 DISCUSSION
PS is very rare, with a prevalence of 0.225%–0.425%, and ELS is less common than ILS, with most ELS located between the left lower lobe and the septum.5 However, the mass in our patient was located on the left side of the diaphragm and was usually asymptomatic or mildly symptomatic.
Due to the presence of the pleura, lobar lung isolation is completely separated from the adjacent normal lung tissue anatomically and forms a separate lung lobe. Ninety percent of ELS is located in the left hemithorax,1 and rarely exists in extrathoracic locations.6 PS receives blood supply from the systemic circulation, most commonly from the thoracic or abdominal aorta, with venous drainage to the pulmonary veins, the azygous vein, the hemiazygos vein, the inferior vena cava, or the right atrium.7 In contrast, the suspected supply artery from the abdominal aorta could be identified in the imaging presentation of this patient. Abdominal PS can present as a retroperitoneal mass or as a cyst, which can be located next to or in communication with the stomach.8 Retroperitoneal pulmonary sequestration (RPS) is a rare cause of retroperitoneal cysts or masses that cannot be well identified.9 As the diagnosis of RPS in this patient was difficult to make preoperatively, the diagnosis could only be confirmed by intraoperative excision of tissue for pathological examination.
The diagnosis of PS can be accomplished with CT angiography. CT angiography provides a noninvasive method compared to catheter aortography, which used to be necessary to determine the arterial supply and make the diagnosis.7 However, in our cases, it was very difficult to make a correct preoperative diagnosis from the imaging presentation. A large part of the reason for this is the rarity of the disease, and our first consideration in clinical work is definitely the most common disease.
Through our systematic review of the relevant literature in recent years (Table 1), we have listed 17 cases in which the masses occurred in different locations, mostly on the left side, and with different clinical presentations. The cases we have listed cover not only patients of all ages but also several common types of disease. Based on these cases, we can generally conclude that the majority of RPS occurs close to the left adrenal region and that the nature of the mass is difficult to confirm by imaging alone, whereas PS occurring at other sites can be correctly diagnosed preoperatively by imaging and that the location and morphologic characteristics of the mass are more reliably defined during surgical exploration for RPS. The diagnosis can be clarified by pathological testing after removal of the mass. On the other hand, regardless of the type of PS, the treatment is currently based on surgical interventions, but if the patient has no obvious symptoms and if the PS has no impact on the patient's life, then leaving the mass untreated could be considered. However, in most cases, surgical resection is needed. Intraoperative care should be taken to avoid damage to nerves and blood vessels and to reduce postoperative complications, and in most cases, the outcome of surgical resection is curative. Reviewing the relevant literature, with the intensive research on PS in recent years, the treatment modalities have become diversified and endovascular treatment is another option available. This less invasive treatment modality has fewer complications.22 Currently a thoracic endograft can be used for PS.23 We can consider thoracic endograft as a first-line treatment because this treatment has the least physiological burden on the patient and the fastest recovery24 or we can perform vascular embolization with Glubran (n-butyl cyanoacrylate), an endovascular technique that may be a less invasive alternative for patients with comorbidities or who have refused surgery.25
| Authors | Published year | Age | Sex | Main symptom | Location | Side | Complication | Resection |
|---|---|---|---|---|---|---|---|---|
| Liu1 | 2012 | 74 | M | Asymptomatic | Adrenal area | Left | – | Yes |
| Sagir Khan3 | 2019 | 67 | F | Asymptomatic | Adrenal area | Left | – | Yes |
| Sagir Khan5 | 2020 | 10 | M | Chest pain and vomiting | Thoracic cavity | Left | Torsion | Yes |
| Petty7 | 2017 | 43 | M | Back pain | Lower lobe | Left | – | Yes |
| Armatys9 | 2005 | 21 | F | Flank pain | Adrenal area | Left | Pneumothorax and a diaphragmatic injury | Yes |
| Marine10 | 2022 | 51 | F | Chest pain and hemoptysis | Lower lobe | Right | – | No |
| Gupta11 | 2017 | 28 | M | Postprandial epigastric pain | Lower lobe | Left | – | Yes |
| Hakiri12 | 2021 | 56 | F | chest pain | Lower lobe | Left | – | Yes |
| Son13 | 2020 | 13 | F | Abdominal pain and fever | Thoracic cavity | Left | Torsion | Yes |
| Jaiswal14 | 2021 | 22 | M | Massive haemoptysis | Lower lobe | Left | – | Yes |
| Baker15 | 1982 | 50 | M | Urinary tract infection | Adrenal area | Left | – | Yes |
| Gomez16 | 2009 | 20 day | M | – | Below the diaphragm, in front of the aorta, and between the right kidney and stomach | Right | - | Yes |
| Gucer17 | 2006 | 5 | M | Abdominal pain and weight loss | Adrenal area | Left | – | Yes |
| Roberts18 | 2000 | 74 | F | Acute gastroenteritis | Adrenal area | Left | – | Yes |
| Furuno19 | 2006 | 41 | M | Flank pain | Adrenal area | Left | – | Yes |
| Kim20 | 2005 | 32 | M | Epigastric discomfort and | Retroperitoneal space | Left | Infection | Yes |
| Yang21 | 2012 | 40 | M | Flank pain | Adrenal area | Left | – | Yes |
4 CONCLUSION
PS can have many different symptoms and can occur in different locations, requiring attention to the differential diagnosis. Similar to the RPS mentioned in this case, we can only make a preliminary diagnosis of a retroperitoneal mass. Reviewing the relevant literature, we learned that bronchogenic cysts,26 retroperitoneal lipomas,27 retroperitoneal dendritic cell sarcoma,28 and retroperitoneal ganglioneuromas29 all have imaging manifestations of retroperitoneal masses and require careful differentiation. The uniqueness of the case is that the symptoms of RPS are only inconspicuous rather than asymptomatic, and because we had so little knowledge of pulmonary isolation, we were unable to make an accurate preoperative diagnosis. The outcome of surgical resection was curative, as determined through long-term postoperative follow-up of the patient.
AUTHOR CONTRIBUTIONS
Tao Wang: Writing – original draft. Zonglei Zhao: Resources; writing – review and editing. Lingqun Kong: Resources; writing – review and editing. Xiaoqin Lyu: Writing – review and editing. Xuefeng Cao: Writing – review and editing. Xingyuan Zhang: Writing – review and editing. Qiangpu Chen: Writing – review and editing.
FUNDING INFORMATION
This research was supported by the Project of Medical and Health Technology Development Program in Shandong Province (202104081018).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
ETHICS STATEMENT
The study was approved by the Ethics Committee of Binzhou Medical University Hospital. Informed consent can be obtained from the clinical database of hospital.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.