A case of central diabetes insipidus associated with cardiac dysfunction
Key Clinical Message
Central diabetes insipidus (CDI) results from a deficiency of arginine vasopressin (AVP) secretion. It is treated by replacement therapy with the synthetic AVP analogue desmopressin. To prevent heart failure in patients with CDI accompanied by cardiac dysfunction, controlling sodium and water intake is essential, using the minimum effective dose of desmopressin.
Introduction
Arginine vasopressin (AVP) is an antidiuretic hormone produced in the hypothalamus and stored in the posterior pituitary gland 1. AVP acts principally on renal collecting tubules to increase water reabsorption through stimulating V2 vasopressin receptors and also has direct roles in regulating cardiovascular function, such as increasing peripheral vascular resistance through V1 vasopressin receptors 2.
Central diabetes insipidus (CDI) is a rare endocrine disorder that results from a deficiency of AVP secretion, and is characterized by a dilute polyuria, plasma hyperosmolality, and relative hypovolemia. CDI is treated by physiological replacement therapy with the synthetic AVP analogue desmopressin (1-deamino-8-D-AVP), a selective V2 vasopressin receptor agonist, to maintain water volume in the body and normal urine output 3, 4. The maintenance dosage of desmopressin is individually based, with the effective minimum dose for Japanese patients being approximately 5–15 μg/day or 120–300 μg/day via intranasal or orally (disintegrating tablet) administration, respectively 5.
Heart failure (HF) is a clinical syndrome characterized by the inability of the heart to supply adequate blood flow to peripheral tissues and organs and can be caused by a variety of structural or functional cardiac disorder 6, 7. Many symptoms of HF, including ankle swelling and pulmonary edema, can result from sodium and water retention and resolve quickly with diuretic therapy.
Although HF is not an uncommon condition, there is little information on the management of CDI accompanied by abnormal cardiac function. We report herein a rare case of a patient with left ventricular (LV) dysfunction with no history of symptomatic HF who developed CDI.
Case Presentation
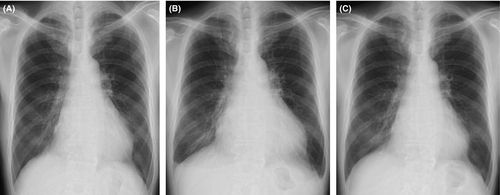
A 63-year-old Japanese man was admitted to our hospital in November 2013 because of thirst and polyuria. His family history was unremarkable. The patient had no smoking habit and would only drink a little alcohol several times a month but never heavily. The patient had been treated with antidepressive agents including paroxetine for depression from 53 to 61 years of age. The patient presented with asymptomatic atrial fibrillation (AF) 8 at a routine examination in April 2009 (at 59 years of age) and visited a local hospital. There were no symptoms or signs of HF such as breathlessness or peripheral edema. An electrocardiogram (ECG) showed AF with a heart rate (HR) of 43 beats/min. A chest X-ray showed an enlarged heart with a cardiothoracic ratio (CTR) of 59% without pulmonary edema, and an echocardiogram showed globally reduced LV wall motion with a LV ejection fraction (LVEF) of 50% (Fig. 1). The patient started anticoagulant therapy with oral warfarin to prevent thromboembolism. His clinical course was uneventful until July 2013, at which point the patient acutely developed thirst and polyuria, drank 4 L of water, and began to void 20 times throughout the day. He also experienced a relapse of his depressive symptoms with appetite and body weight (BW) loss in October 2013 and visited a psychiatrist. He was diagnosed with recurrent depression and restarted antidepressive therapy with oral paroxetine. Thereafter, the patient was referred to our hospital because of his persistent thirst and polyuria and was admitted for further detailed examinations.
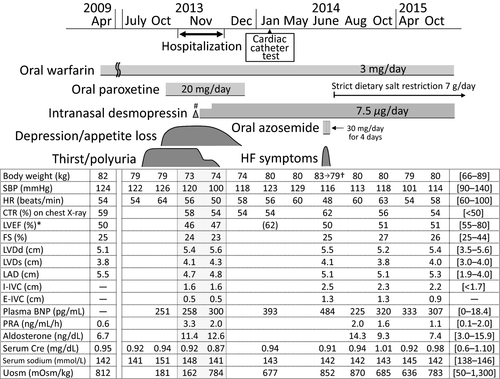
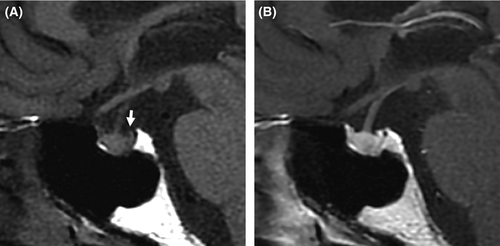
At physical examination, the patient was 189 cm tall and weighed 73 kg, and his body temperature, blood pressure (BP), and oxygen saturation by pulse oximeter (SpO2) on room air were 36.4°C, 104/60 mmHg, and 99%, respectively. A mild systolic regurgitant murmur was audible, but no jugular vein swelling, chest rale, or peripheral edema was detected. The volume of urine (3600 mL/day) throughout 1 day was large. Laboratory findings (Table 1) showed high levels of serum sodium and chloride, high plasma osmolality (Posm) and plasma renin activity (PRA), low urinary osmolality (Uosm), and undetectable plasma AVP. His thyroid function was normal, while levels of plasma brain natriuretic peptide (BNP) were high. A desmopressin stimulation test showed increased Uosm along with reduced volume of urine and increased levels of urinary aquaporin-2 (Table 2) 11. Dynamic tests for secretion of anterior pituitary hormones showed normal releases of all of prolactin, thyroid-stimulating hormone, growth hormone, adrenocorticotropic hormone, cortisol, luteinizing hormone, and follicle-stimulating hormone. Magnetic resonance imaging of the brain showed no morphological abnormalities, but the physiological high-intensity signal, which would be expected in the posterior pituitary on T1-weighted plane images, was undetectable (Fig. 4), indicating a diagnosis of CDI. Tests for autoantibodies against the anterior and posterior lobes of the pituitary gland were negative.
| Hematology | |
| Red blood cells | 507 × 104/μL (427–571) |
| Hemoglobin (g/dL) | 16.2 (12.4–17.2) |
| Hematocrit (%) | 47.8 (38.7–50.3) |
| White blood cells | 5300/μL (4000–9000) |
| Platelets | 17.4 × 104/μL (12.0–30.0) |
| Chemistry | |
| Total protein (g/dL) | 7.0 (6.7–8.3) |
| Albumin (g/dL) | 3.8 (3.8–5.3) |
| Aspartate aminotransferase (IU/L) | 28 (13–33) |
| Alanine aminotransferase (IU/L) | 12 (8–42) |
| Blood urea nitrogen (mg/dL) | 11.1 (8.0–20.0) |
| Creatinine (mg/dL) | 0.92 (0.60–1.00) |
| Sodium (mmol/L) | 148 (138–146) |
| Potassium (mmol/L) | 4.6 (3.5–4.7) |
| Chloride (mmol/L) | 111 (98–108) |
| Calcium (mg/dL) | 9.0 (8.8–10.3) |
| Phosphorus (mg/dL) | 3.4 (2.5–4.7) |
| Total cholesterol (mg/dL) | 213 (130–220) |
| Triglycerides (mg/dL) | 100 (50–130) |
| Fasting plasma glucose (mmol/L) | 4.4 (3.9–6.1) |
| HbA1c (NGSP) (%) | 5.7 (4.6–6.2) |
| C-reactive protein (mg/dL) | 0.09 (<0.30) |
| Thyroid-stimulating hormone (μU/mL) | 0.81 (0.35–4.94) |
| Free thyroxine (ng/dL) | 0.72 (0.70–1.80) |
| Free triiodothyronine (pg/mL) | 2.09 (2.00–4.90) |
| Brain natriuretic peptide (pg/mL) | 226.9 (<18.4) |
| Plasma osmolality (mOsm/kg) | 304 (275–290) |
| Plasma arginine vasopressin (pg/mL) | <0.8 (>4.0)a |
| Plasma renin activity (ng/mL/h) | 3.3 (0.1–2.0) |
| Serum aldosterone (ng/dL) | 11.4 (3.0–15.9) |
| Urinary osmolality (mOsm/kg) | 162 (50–1300) |
| Urinalysis | |
| Specific gravity | 1.005 (1.006–1.020) |
| pH | 7.0 (4.5–7.5) |
| Glucose | negative |
| Protein | negative |
| Occult blood | negative |
| White blood cells | negative |
- Blood samples were taken in the morning with the patient in the supine position. The reference range for each parameter is shown in parentheses.
- a The reference range for plasma arginine vasopressin was calculated from the patient's plasma osmolality, based on the published approximate calculation formula 9.
- NGSP, National Glycohemoglobin Standardization Program 10.
| Time (min) | |||||
|---|---|---|---|---|---|
| 0 | 30 | 60 | 90 | 120 | |
| Urine volume (mL/30 min) | 60 | 58 | 34 | 22 | 22 |
| Urinary osmolality (mOsm/kg) | 215 | 396 | 493 | 517 | 571 |
| Urinary aquaporin-2 (ng/mL) | 0.44 | – | – | – | 6.38 |
| Plasma arginine vasopressin (pg/mL) | <0.8 | – | – | – | – |
| Serum sodium (mmol/L) | 148 | – | 146 | – | 146 |
| Plasma osmolality (mOsm/kg) | 303 | – | 300 | – | 300 |
- The patient refrained from eating and drinking 2 h before and for the duration of the test. Urine samples were taken at 0 (just before), 30, 60, 90, and 120 min after desmopressin (10 μg) was administered nasally.
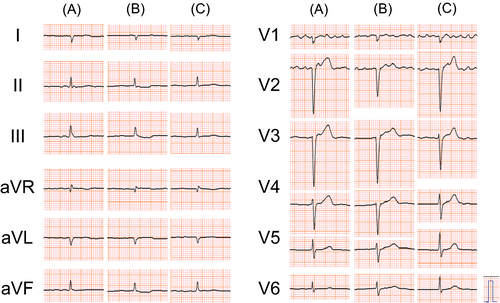
As to the patient's cardiac function, ECG showed chronic AF with a HR of 54 beats/min (Fig. 2A), and a chest X-ray showed an enlarged heart (Fig. 3A). An echocardiogram showed globally reduced wall motion (LVEF 46%) accompanied by an akinetic region at the posterior wall, with normal wall thickness, mild left atrial dilatation, and mild mitral regurgitation.
The patient started treatment for CDI with intranasal desmopressin at 5 μg/day on Day 6 of admission and experienced some decreases in thirst, polydipsia, and dilute polyuria (Fig. 1). The dose of desmopressin was titrated to 7.5 μg/day on Day 10, and thereafter, he had almost complete improvements in his CDI symptoms. His urinary volume and osmolality throughout Day 12 of admission were 1100 mL/day and 784 mOsm/kg, respectively, and his urinary frequency was 5 times/day. An echocardiogram performed on Day 13 of admission showed cardiac parameters comparable to those at the time of admission. To avoid dehydration or water intoxication and heart failure, the patient was instructed to ingest water freely for thirst in the range of 1500 mL/day and to take sodium <7 g/day and was discharged 14 days after admission.
The patient experienced improvements in his depressive symptoms, regained his appetite and BW, and discontinued antidepressive drugs on the basis of his psychiatrist's judgment in December 2013 (Fig. 1).
A detailed evaluation of cardiac function was performed in January 2014. A chest X-ray showed an enlarged heart with a CTR of 54%, and ECG showed chronic AF with a HR of 58 beats/min, both of which were comparable to those performed in November 2013 before therapy with desmopressin. Myocardial scintigraphy with thallium 201 showed decreased uptake in the posterior wall of the left ventricle without redistribution. Cardiac catheterization used with coronary arteriography showed no significant coronary artery stenosis or dilatation. Left ventriculography showed a relatively preserved global LV wall motion with a LVEF of 62%, and an akinetic region in the inferior-to-posterior wall of the left ventricle was found. His endo-diastolic volume, endo-systolic volume, stroke volume, and cardiac output were 222, 83, 138 mL, and 12.7 L/min, respectively. As both the patient's BP and HR were relatively low, he did not receive medication for LV dysfunction, such as renin-angiotensin-aldosterone system inhibitors or beta-blockers 6, 7. He continued to receive anticoagulant therapy with warfarin.
The patient came to consume slightly larger amounts of salt in Spring 2014 by eating Japanese pickles. In June of the same year, the patient visited our hospital after several days of fatigue, lower-extremity edema, and a 4-kg BW gain. His BW, body temperature, BP, and SpO2 on room air were 83 kg, 36.2°C, 116/80 mmHg, and 97%, respectively. No chest rales or heart murmurs, except for preexistent mild systolic regurgitant murmur, were detected, but bilateral pedal edema was observed. Laboratory findings revealed a normal complete blood count, normal serum electrolytes, and normal renal, liver, and thyroid functions, but his plasma BNP level (484.0 pg/mL) was higher than that measured previously (Fig. 1). A troponin test was negative. ECG showed chronic AF and a HR of 48 beats/min (Fig. 2B). A chest X-ray detected cardiomegaly with a CTR of 62% and bilateral mild pulmonary effusion (Fig. 3B). An echocardiogram showed mildly reduced LV wall motion with a LVEF of 50%. He was diagnosed with HF and started oral azosemide at 30 mg/day under a continued 7.5 μg/day of intranasal desmopressin. The patient experienced rapid improvements in his fatigue and lower-extremity edema with sufficient diuresis, and his BW returned to 79 kg within 4 days (Fig. 1). He then discontinued azosemide on his own judgment. He underwent treatment with strict dietary salt restriction (7 g/day) and adequate water intake (1500 mL/day) without resuming diuretics in combination with desmopressin (7.5 μg/day) replacement for CDI.
His subsequent clinical course during desmopressin replacement was uneventful, without recurrence of HF (Figs 1, 2C and 3C).
Discussion
The patient with a 4-year duration of mild LV dysfunction but no history of symptomatic HF developed CDI and started treatment with a minimum fixed dose of desmopressin (7.5 μg/day) that was necessary to relieve his thirst and dilute polyuria. He did not exhibit hyponatremia or low PRA, but 7 months after the same desmopressin replacement, he exhibited symptomatic HF without verifiable causes except for excess salt intake, which resolved rapidly during several days of oral diuretics under continued desmopressin therapy. Following strict restriction of dietary salt and water, he had no recurrent symptomatic HF.
The primary stimulus for AVP secretion under normal physiological conditions is an increase in Posm. Conversely, an excessive administration of desmopressin can cause decreased Posm and hyponatremia in association with excessive water retention 3, 4. In the present case, the patient never exhibited hyponatremia during the course of the minimum effective dose of desmopressin therapy for his CDI (Fig. 1). Additionally, PRA, which would decrease if the extracellular fluid volume increases 12, was not low during that period. These findings suggest that excessive retention of water in the body was not clinically detected by measuring electrolytes, Posm, or PRA during desmopressin treatment in our patient.
Few studies have investigated the effects of AVP deficiency or desmopressin replacement on cardiac performance in patients with abnormal cardiac function. However, a study on patients with CDI without a cardiac disorder suggested that AVP deficiency induces reversible changes in cardiac function, such as increased HR and LV contractility in association with relative hypovolemia 13. That study also suggested that AVP deficiency induces subclinically altered LV diastolic function that persists even after treatment with adequate doses of desmopressin. In the present case, the echocardiogram systolic parameters including LVEF and fractional shortening were similar before the development of CDI and after desmopressin therapy (Fig. 1). However, other cardiac parameters such as the inferior vena cava diameter 14 and plasma levels of BNP 15 were large or high especially after commencement of desmopressin therapy and before strict dietary sodium restriction, compared with those during the other period. Taken together, these findings suggest that our patient probably became more susceptible to HF after he developed CDI and underwent the desmopressin therapy than had been the case previously.
The patient had chronic AF with bradycardia and showed echocardiogram findings of normal LV diameter and wall thickness, mild left atrial dilatation, and mild mitral valve regurgitation, all of which remained almost unchanged during the course of his LV dysfunction >6 years (Fig. 1). Myocardial scintigraphy with thallium 201 showed decreased uptake in the posterior wall of the left ventricle, and a left ventriculography showed an akinetic region at that area in the absence of coronary artery lesion. Based on these results, although his mitral valve regurgitation or AF might have contributed in part to his LV dysfunction 2, the major etiology of the patient's LV dysfunction remains unclear. A careful check of cardiac function along with the course of desmopressin therapy for CDI was required in this case.
Central diabetes insipidus can be caused by the destruction or degeneration of neurons that originate in the supraoptic and paraventricular nuclei of the hypothalamus. Known causes of these lesions include tumors, local inflammatory, autoimmune or vascular diseases, trauma, and surgery, but many CDI cases are of unknown cause 1. The present patient developed acute thirst and polyuria due to AVP deficiency without verifiable causal factors (Fig. 4) and, thus, his CDI was idiopathic.
In conclusion, we reported a patient with CDI and cardiac dysfunction who developed HF during treatment with a minimum effective dose of desmopressin replacement. This is a single case study and thus does not provide a specific therapeutic proposal, including dosage calculation for desmopressin, on the management of CDI associated with cardiac dysfunction. However, this case suggests that patients with cardiac dysfunction may become more susceptible to HF after they develop CDI and undergo desmopressin therapy than previously. Therefore, to prevent HF in patients with CDI accompanied by abnormal cardiac function, it is essential to control sodium and water intake using the effective minimum dose of desmopressin.
Acknowledgment
We thank the medical laboratory technicians of Nagaoka Red Cross Hospital for their helpful technical support.
Consent
Written informed consent was obtained from the patient for publication of this case report.
Conflict of Interest
None declared.




