Triad of Cerebellar Hematoma, Obstructive Hydrocephalus and Non-Traumatic Subarachnoid Hemorrhage in a Hypertensive Patient: A Case Report and Literature Review
Funding: The authors received no specific funding for this work.
ABSTRACT
We are reporting the case of a hypertensive middle-aged adult with a triad of right cerebellar hematoma, acute obstructive tri-ventricular hydrocephalus, and spontaneous subarachnoid hemorrhage. A 45-year-old male with a 3-year history of uncontrolled hypertension presented with a severe thunderclap headache, left lower facial weakness, left symmetrical hemiparesis, ataxia, dysdiadochokinesis, signs of intracranial hypertension, grade III hypertension, and a Glasgow coma score (GCS) of 13/15 with a loss of spatial and temporal orientation. A non-contrast-enhanced cerebral CT scan showed a right cerebellar hematoma complicated by a diffuse cerebral edema, a tri-ventricular obstructive hydrocephalus from compression of the fourth ventricle, and a subarachnoid hemorrhage. A conservative management approach was adopted by the neurosurgeon based on the high GCS on entry, slight improvement in the signs of intracranial hypertension and blood pressure, and the lack of a neurosurgical technical platform to manage the case surgically. On his fifth day of admission, he was transferred to the intensive care unit (ICU) following a significant deterioration in his GCS (8/15), despite improvement in his blood pressure (BP) and intracranial pressure. He unfortunately passed away in the ICU 24 h later following a cardiorespiratory arrest. The exact etiology of this extremely rare occurrence remains obscure, and the management is all the more challenging, especially in a setting like ours where neurosurgical services are still underdeveloped. This case highlights the need not only for the establishment of proper neurosurgical services in our setting but also for standardized guidelines for the management of these cases.
Summary
- The triad of cerebellar hematoma, hydrocephalus, and subarachnoid hemorrhage, though extremely rare, presents a tremendous life threat.
- Guidelines on how to manage such cases remain inconclusive due to existing controversies. The absence of proper neurosurgical services in low-resource settings equally makes the management of such cases extremely challenging.
1 Introduction
Cerebellar hemorrhages account for 10% of all intracerebral hemorrhages and 15% of cerebellar strokes. Cerebellar hemorrhages are associated with high morbidity and mortality [1, 2]. Due to the tightness of the posterior cranial fossa, even a minor cerebellar hemorrhage can form a hematoma and compress the brainstem, resulting in potentially life-threatening consequences [3, 4]. These life-threatening consequences include acute obstructive hydrocephalus due to compression of the cerebral aqueduct and/or 4th ventricle, diffuse cerebellar edema with mass effect increasing the intracranial pressure, and also brainstem compression with transforaminal and upward transtentorial herniation [5, 6]. Likewise, the simultaneous occurrence of aneurysmal subarachnoid hemorrhage (SAH) and remote forms of intracerebral hemorrhages such as cerebellar hemorrhage though possible, is very uncommon, with only four cases reported worldwide over the years [7, 8]. It is therefore even more rare to have a triad of cerebellar hemorrhage, obstructive hydrocephalus, and subarachnoid hemorrhage (SAH), even though severe hypertension and the presence of vascular anomalies like aneurysms could be the binding factor for this triad to occur [9]. Standard management requires a neurosurgical team, a stroke unit team, or neuro-intensive care unit (neuro-ICU) with surgical treatment often viewed as a necessity. However, such standards remain difficult to achieve even in secondary healthcare facilities due to the substandard facilities and lack of both human and material resources [10]. We are reporting the case of a hypertensive middle-aged adult with a triad of right cerebellar hematoma, acute obstructive tri-ventricular hydrocephalus, and spontaneous subarachnoid hemorrhage with diffuse cerebral edema at Buea Regional Hospital, Cameroon.
2 Case History
2.1 Patient Information
He is a 45-year-old hypertensive male diagnosed 3 years prior with inconsistent compliance to his treatment who presented initially at a primary healthcare facility with an elevated blood pressure of 243/142 mmHg associated with generalized body weakness, dizziness, blurred vision, and 3 episodes of copious projectile vomiting, which started 8 h prior to consultation. Three hours later, he had a severe throbbing headache of sudden onset, radiating to the neck, without exacerbating and alleviating factors. He graded the intensity of the headache as a 10 out of 10 on the verbal numerical scale and even referred to it as “the worst headache of his life.” Following evaluation, a diagnosis of hypertensive emergency was made, and intravenous boluses of 2.5 mg of nicardipine were administered every 30 min for 2 h (4 doses), but the blood pressure refractorily stood at 224/140 mmHg. Persistence of the symptoms together with the failure to bring down the blood pressure to reassuring values, and the ultimately the lack of a technical platform to manage such a case, prompted his immediate referral 10 h later to Buea Regional Hospital (BRH), one of the two referral hospitals of the Southwest region of Cameroon. BRH also counts among its ranks the sole attending neurosurgeon of the Southwest region of Cameroon. Upon arrival at BRH, he developed a left facial weakness and an inability to stand on both feet and/or maintain his balance.
He consumes 7.7 units of alcohol a week and denies any history of passive or active smoking. There was no history of myocardial infarction, atrial fibrillation, or stroke. He is not diabetic and denies any family history of diabetes mellitus or malignancy of any form.
2.2 Clinical Findings
On physical examination, he was conscious but lethargic with a Glasgow coma score (GCS) of 13/15 (E = 3, V = 4, M = 6). His blood pressure was 228/140 mmHg, pulse rate was 84 beats per minute and regular, respiratory rate was 24 breaths per minute and regular, oxygen saturation (SaO2) was 100%, and axillary temperature was 37.2°C. His conjunctivae were pink, and sclerae were anicteric. On neurological assessment, his higher mental functions were all intact except for a lack of spatial and temporal orientation. There were no signs of meningeal irritation (Brudzinski and Kernig signs were negative). His pupils were symmetrically reactive to light. There was no eye deviation and no nystagmus. There was a left lower facial paresis with an intact left occipitofrontalis muscle (intact left upper facial movement). Motor examination revealed a mild symmetrical hemiparesis of 4/5 of the left side of his body. Muscle tone and bulk were normal. The biceps, patellar, and ankle reflexes were assessed and normal. Sensory function was preserved. Cranial nerves were all assessed and intact. The Romberg test was positive, while the Rinne and Weber tests were normal. The finger-to-nose test was abnormal, and there was dysdiadochokinesis. On cardiac auscultation, the first and second heart sounds were normal and regular on all four cardiac auscultatory areas. There were no added sounds and no murmurs. Breath sounds were vesicular with no added sounds. Gastrointestinal and musculoskeletal examination was unremarkable.
2.3 Diagnosis
Hypertensive emergency with target organ damage to the brain. Emergency being a cerebrovascular accident (CVA) complicated by a raised intracranial pressure (ICP).
2.4 Diagnostic Assessment
A non-contrast-enhanced cerebral CT scan done 36 h after the onset of symptoms revealed a right cerebellar hemorrhagic CVA (Figure 1) complicated by a diffuse cerebral edema, a tri-ventricular hydrocephalus due to an important mass effect on the 4th ventricle (Figure 2), and subarachnoid hemorrhage (Figure 3). However, the exact size and volume of the hematoma were not quantified by the radiologist.
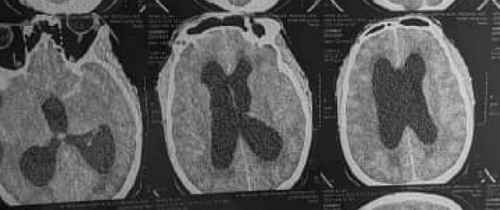
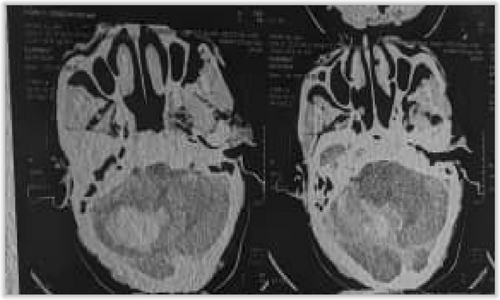
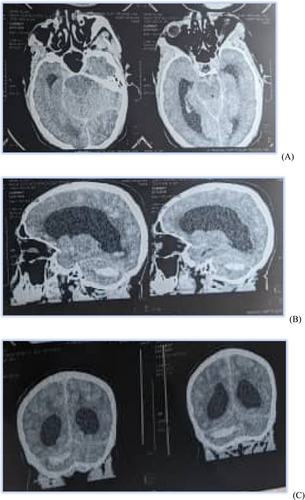
An electrocardiogram (ECG), serum electrolytes, lipid profile, urinalysis, urea, and creatinine were all requested but unfortunately not done due to financial constraints.
2.5 Timeline of the Current Episode
He was admitted to the male medical unit on intravenous (IV) nicardipine 10 mg administered at a rate of 5 mL/min using an electric syringe pump and 1 tablet BID of labetalol 200 mg with close monitoring of blood pressure every 30 min. Mannitol 20% at a dose of 200 cc was administered 6 hourly to control intracranial pressure. Nefopam 20 mg IV 1 vial 12 hourly, paracetamol 1 g IV 1 vial 6 hourly slow infusion, and normal saline 0.9% 500 cc 12 hourly were also administered. His vital signs and level of consciousness were monitored every 30 min. Oxygen saturation was monitored continuously, and the urine output was recorded every 4 hourly. A neurosurgical evaluation was also booked for further management modalities to be added. Following neurosurgical evaluation, a conservative management approach was adopted. This was based on the high GCS on entry and a slight improvement in blood pressure initially observed. The lack of an adequate neurosurgical technical platform limited surgical options and thus also contributed to the adoption of a conservative approach. Besides, the refusal of early referral to a referral hospital with proper neurosurgical services by the family of the patient due to distance and financial constraints further limited the management options and thus greatly impacted the prognosis negatively.
2.6 Follow-Up and Outcome of Interventions
Progress in the ward was marked by the development of agitation and restlessness on day 3 of admission, for which he was placed on phenobarbital 100 mg slow IV 12 hourly and mexazolam 1 mg 1 tablet BID. The intensity of the headache improved from 10/10 on entry to 7/10 on day 3, and the projectile vomiting subsided. His GCS gradually dropped over the course of his admission from 13/15 (E3V4M6) on entry to 10/15 (E3V3M4) on day 3. By the end of day 3, there was failure to control his blood pressure using intravenous nicardipine and labetalol, with his blood pressure fluctuating between 240/142 mmHg and 184/121 mmHg. The dose of nicardipine was thus stepped up to 6 mL/min using the electric syringe pump, and the perindopril 10 mg + amlodipine 10 mg combination of 1 tablet daily was added to his treatment.
By day 5 of admission, following the failure to control his blood pressure, the worsening of his state of consciousness (GCS 8/15—E2V2M4), the persistent agitation, restlessness, and signs of raised intracranial pressure, he was transferred to the intensive care unit (ICU) for better management. Unfortunately, after another episode of agitation, he manifested signs of respiratory distress and later on suffered a cardiorespiratory arrest that proved fatal. He died 24 h after his transfer to the ICU.
3 Discussion
3.1 Pathophysiology
The occurrence of these three simultaneous conditions remains a rare sight, and the exact cause of this phenomenon is definitely unknown. However, we suggest the following pathophysiological mechanism:
Brainstem compression with direct mass effect and compression of the 4th ventricle itself or the cerebral aqueduct by a cerebellar hematoma could result in obstructive hydrocephalus [11]. This is made possible by the tightness of the posterior cranial fossa providing a clear medium for the formation of hematomas, which, if formed, could compress posterior cranial fossa structures such as the cerebellum, brainstem, cerebral aqueduct, and fourth ventricle with ease and give rise to the above complications. With regard to SAH, its direct connection to cerebellar hemorrhagic CVA as a complication is unlikely, as it usually results more from traumatic mechanisms [9, 11]. SAH could also result from non-traumatic causes such as arteriovenous malformations, for example, aneurysms; however, their respective site of occurrence does not favor the possibility of their simultaneous occurrence. Moreover, in the setting of aneurysms, SAH develops after rupture of the latter, principally as a result of either uncontrolled hypertension (hypertensive emergencies) or trauma [9]. Nevertheless, anatomically, the apex of the fourth ventricle extends into the base of the cerebellum with its external germinal layer in close proximity to the cerebellum. Also, with the fourth ventricle housing the foramina of Magendie and Luschka, which connect the fourth ventricle to the subarachnoid space, it is safe to assume that bleeding into the fourth ventricle could extend into the subarachnoid space and eventually culminate in subarachnoid hemorrhage. Therefore, in the setting of the close proximity of the cerebellum and the fourth ventricle, if the external germinal layer of the fourth ventricle were to be breached, then hemorrhage from the cerebellum could easily extend into the fourth ventricle and later into the subarachnoid space, eventually causing subarachnoid hemorrhage.
Hypertension is the main cause of cerebellar hematomas, with hypertensive cerebellar hemorrhage constituting 5 to 10% of all hypertensive intracranial hemorrhage cases worldwide [12]. Uncontrolled hypertension also remains the most supported etiologic mechanism for the SAH of the index case. Consequently, the simultaneous occurrence of aneurysmal SAH and cerebellar hemorrhage, though possible, is rare [7, 8]. It is even more rare in the setting of a triad of cerebellar hemorrhage, obstructive hydrocephalus, and SAH. Nevertheless, the concomitant presence of severe hypertension and/or vascular anomalies like aneurysms, as well as the occurrence of the hemorrhage in the posterior cranial fossa (cerebellum and its relation to the fourth ventricle), could likely be the binding factor for this triad to occur [9].
3.2 Management
The management of these conditions individually or collectively could be either conservative or surgical depending on several factors that must be taken into consideration. This is especially the case when they occur simultaneously, like for the index case. In that line, when surgery is indicated, the decisions regarding surgical treatment are determined by factors such as the size of the hematoma, the presence of hydrocephalus, the degree of basal cisternal and/or fourth ventricular compression, and the location of the hematoma [13-15].
3.3 Size of Hematoma
According to the American Heart Association/American Stroke Association (AHA/ASA), surgery is recommended for patients who showed neurological deterioration and a maximum hematoma diameter of greater than 3 cm or a cerebellar hematoma volume of greater than 10 mL [16]. The size of the hematoma was observed to be strongly correlated with outcomes in some series [16, 17]. It was even demonstrated to have a strong correlation between the clinical course and the volumetrically calculated size of the hematoma on CT scans [16, 17]. In that line, in a study carried out in Korea in 2015, external ventricular drainage (EVD) alone was performed in patients with small hematomas who exhibited progressive deterioration in their GCS associated with hydrocephalus caused by IVH or 4th ventricle obliteration [12]. Patients with cerebellar hematomas less than 3 cm and without hydrocephalus, who were usually conscious and had good GCS scores, were treated conservatively [18]. Besides, the depressed level of consciousness in cases of cerebellar hematomas has been largely attributable to hydrocephalus, direct brainstem compression by the hematoma, surrounding swelling, or both [3, 4]. The main controversy, however, resides in deciding which cases require surgical evacuation of the hematoma versus other options, such as ventricular drainage only or conservative treatment [3, 4]. Surgical evacuation of posterior fossa hematomas is associated with great morbidity and mortality risks [16, 19], and simple drainage of the hydrocephalus may be ineffective [19]. Therefore, in cases of associated raised intracranial pressure, surgical evacuation should be considered, with a careful assessment of pros and cons. In addition to that, because the clinical course is variable in some cases, the timing of such treatment should be carefully considered too [16, 19]. However, in the index case, the size of the hematoma was not specified by the radiologist. This could have also posed a major challenge in the evaluation of the management options of the patient.
3.4 Compression of the 4th Ventricle
The degree of compression of the 4th ventricle is classified into three groups: normal, partial compression, and complete obstruction, based on the computed tomographic (CT) appearance of the fourth ventricle [12].
Grade I, normal size and configuration, located in the midline (if intraventricular hemorrhage is present, cerebrospinal fluid (CSF) is still visible in the fourth ventricle). If the hematoma has ruptured into the fourth ventricle, the presence of CSF surrounding the incompressible intraventricular clot should be noted and included as Grade I [12, 18] (Figure 4A);
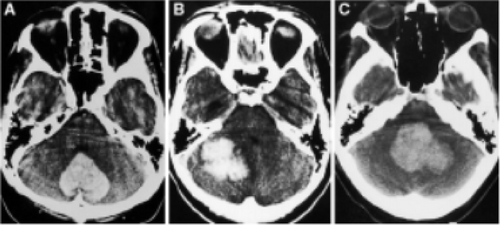
Grade II, partially compressed or distorted, shifted to the contralateral side (in cases of unilateral hematomas) [12, 18] (Figure 4B);
Grade III, complete obliteration, with anterior shift distorting the brainstems and obliterating the prepontine space (even if the fourth ventricle is partially compressed) [12, 18] (Figure 4C).
In all cases of Grade III fourth ventricular compression, evacuation of the hematoma is recommended, even for patients with a good level of consciousness, on the presumption that these patients could be at risk of subsequent deterioration due to brainstem compression [12, 18] (Figure 5).
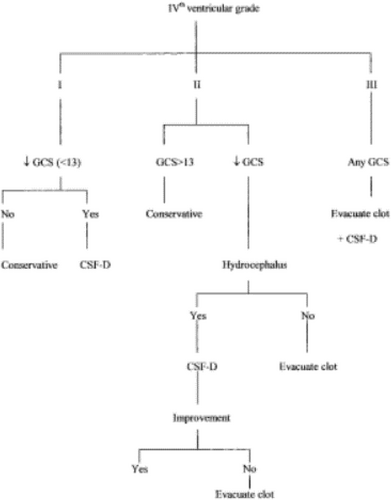
In cases of patients with Grade II fourth ventricular compression like for the index case, whose GCS scores are 13 and above, initial monitoring is recommended. However, if deterioration in the level of consciousness occurs, then ventricular drainage of hydrocephalus should be performed as soon as possible. If the level of consciousness fails to improve after ventricular drainage or in the absence of significant ventriculomegaly, then evacuation of the hematoma is indicated [12, 18] (Figure 5).
In cases with Grade I fourth ventricular compression, hematoma evacuation is considered to be unnecessary, and ventricular drainage is required only if hydrocephalus develops, resulting in impaired consciousness [12, 18] (Figure 5).
Nevertheless, it is important to keep in mind that fourth ventricular compression grades should be considered alongside other factors, especially GCS scores, during the management of cerebellar hematomas [12, 18].
3.5 Presence of Hydrocephalus
Hydrocephalus occurs as a result of the mass effect of the hematoma on the fourth ventricle, the aqueduct, or secondary to IVH, eventually obstructing CSF flow [10]. Hydrocephalus has been observed in several case series to be one of the most important factors determining outcomes of cerebellar hematoma cases [10, 12]. The presence of hydrocephalus has been associated with poor prognoses [10, 12, 20]. The presence of hydrocephalus, therefore, has been used as a strong indicator for surgical treatment and prognosis [10, 12, 20]. It was also stated that in the setting of a cerebellar hematoma or other forms of intracranial hematomas and hydrocephalus, initial treatment should be directed toward the drainage of hydrocephalus before direct surgical evacuation of a hematoma [10, 12, 20]. This is because the severity of the hydrocephalus has a direct and greater impact on the degree of brainstem compression, as it has a faster and greater expansion rate than a hematoma, and it is closer to the brainstem too. It therefore requires every effort geared toward draining the CSF before the hydrocephalus exacerbates the brainstem compression and worsens the prognosis due to the very high intracranial pressure that would result.
However, in some cases of ventricular compression with hydrocephalus, even the simplest ones, such as Grade I or II ventricular compression with hydrocephalus, ventricular drainage alone may be ineffective [10, 12, 20]. In the presence of a large posterior fossa mass, for example, ventricular drainage alone is thought to carry a risk of upward coning [12]. Therefore, patients who are initially treated with external ventricular drainage may subsequently require craniectomy and evacuation of the lesion. However, this usually fails to reverse the deterioration in some cases, keeping in mind that direct brainstem compression plays the predominant role in their poor neurological state [10, 12, 20]. There is therefore the need for methods to identify the appropriate initial treatment before further irreversible neurological damage occurs, because when surgery is indicated. Hence, controversy still exists regarding whether ventricular drainage only, evacuation of the hematoma, or both procedures should be performed [10, 12, 20]. Nevertheless, initial management of the hydrocephalus before the evacuation of hematoma remains the preferred approach in most cases [10, 12, 20].
3.6 Cause of Death
The brainstem comprises the midbrain, pons, and medulla oblongata. The medulla oblongata contains the vasomotor and respiratory centers, which control heart rate and blood pressure and respiratory rhythm and rate, respectively [21]. The pons contains the apneustic and pneumotaxic centers, which control the depth of respiration. Death in this condition will commonly occur in the form of cardiac and/or respiratory arrest as a result of pressure and compression of the brainstem (medulla oblongata in particular) with compromise of these cardiorespiratory centers [21]. That is why it is primordial to address the condition early enough with the appropriate team and approach because the patient's deterioration speed is very much proportional to the degree of brainstem compression, with outcomes such as coma or death being very likely.
3.7 Prognosis
With regards to possible prognostic risk factors for early outcome, it has been demonstrated that initial neurological status using the GCS score is the only significant predictor of 30-day mortality after a cerebellar hemorrhagic CVA. Patients with an initial GCS score < 10 had an in-hospital mortality of about 89% and suffered an unfavorable outcome (mRS ≥ 3) in approximately 97% of cases [22]. In another study, it was found that a GCS score of < 8 and radiological evidence of acute hydrocephalus or IVH are the most significant predictors of early mortality [23].
Hydrocephalus is a clinical emergency in patients with cerebellar hemorrhagic CVA and has been described in many studies as a significant risk factor for unfavorable outcomes. However, its early diagnosis and rapid treatment have a positive impact on outcome [24, 25]. Furthermore, the location of the hematoma is believed to have a strong role to play, with midline (vermian) hematomas being more likely to cause hydrocephalus than lobar ones [22]. However, obstructive hydrocephalus can result from the presence of intraventricular hemorrhage, which explains the occasional lack of correlation between the degree of fourth ventricular compression and hydrocephalus. Therefore, in the setting of hydrocephalus without direct signs of fourth ventricular compression, intraventricular hemorrhage should always be ruled out as a possible etiology, as it can not only cause obstructive hydrocephalus but is also associated with significantly higher mortality rates [22].
Death may also occur among patients treated conservatively (2), as observed in a case series where outcomes after nonsurgical management were variable, with mortality rates between 9% and 75% in high-risk patients in many series [26-28]. These deaths could possibly be attributed to the deterioration of those patients or even the presence of comorbidities [26-28].
It was reported in a series that severe compression of the fourth ventricle (Grade III) on the initial CT scans has been associated with low GCS scores at presentation and poor outcomes. Furthermore, identification of this radiological sign could serve as a predictor of subsequent clinical course, as seen with the 43% of initially conscious (GCS 14–15) patients with Grade III ventricular compression who experienced rapid deterioration into coma before admission to our neurosurgical department [22].
Furthermore, the fourth ventricular appearance grades have been shown to be well correlated with hematoma volumes and clinical courses. Areas of swelling surrounding hematomas and exerting mass effect could also affect the fourth ventricular grade. This indicates the possible value of this radiological sign as an indicator of the degree of brainstem compression. It is less affected by the degree of hydrocephalus than quadrigeminal cisternal compression and therefore may facilitate selection of the surgical procedure, that is, hematoma evacuation versus ventricular drainage only [28].
Finally, the presence of SAH is another factor associated with poor outcomes. Individually, SAH is associated with great morbidity and mortality, especially in low-resource settings [22]. Thus, when associated with other severe conditions such as cerebellar hemorrhagic CVA, hydrocephalus, and cerebral edema, as is the case for the index case, the chances of survival become extremely slim, as each of these conditions already carries enormous mortality risks [22]. Nevertheless, its presence is not an indication for surgical management of the hematoma, as the major part of its management is medical (conservative). Therefore, in the above scenario, medical management of the subarachnoid hemorrhage can be initiated and done while managing the hematoma based on factors such as hematoma size, level of consciousness, and presence of hydrocephalus.
3.8 Challenges Regarding Management in Low-Resource Settings
The existing controversies regarding the management of such a condition, especially in terms of management options and prognosis, continue to pose a great challenge even in referral hospitals in our setting. The existing literature regarding the best approach to this case is relatively sparse and inconclusive. When the primary mechanism of neurologic deterioration seems to result from direct brainstem compression, the literature suggests that definitive surgical management requires suboccipital craniectomy with evacuation of the hematoma or resection of infarcted cerebellar tissue to relieve the directly offending mass effect on the brainstem [11]. In addition, when deterioration seems to result from obstructive hydrocephalus alone and there is no obvious evidence of direct brainstem compression by clinical examination or imaging findings, it remains unclear whether ventriculostomy alone may be adequate for the prevention of further deterioration or whether definitive resection should still be performed to relieve ventricular obstruction and brainstem compression [11].
If hydrocephalus seems to be the main condition responsible for deterioration, a temporizing ventriculostomy can be considered. If a patient improves, no further surgical therapy may be needed. If the patient does not improve, then a definitive posterior fossa decompressive suboccipital craniectomy should be urgently performed [11]. Because craniectomy carries an elevated risk of morbidity and mortality, especially posterior fossa craniectomy, when the patient's clinical course of deterioration is relatively slow for several hours, a strategy of staged procedures with initial ventriculostomy followed by craniectomy if necessary is reasonable [11]. However, with more catastrophic and rapidly evolving neurologic deterioration for less than an arbitrary 8-h limit, and when the risk associated with craniectomy is relatively low, such as in younger individuals, then primary craniectomy should probably be the treatment of choice [11].
These existing controversies delay decision-making and narrow the chances of survival of the patient even more. There is therefore the need for methods to identify the appropriate initial treatment before further irreversible neurological damage, as well as human resources, logistics, and clear and standard management guidelines to address such cases [10]. However, starting neurosurgery services in a rural setting remains a very challenging task requiring infrastructure and resources, particularly linked to neurosurgical services. Major challenges commonly seen include the absence of a neurosurgeon, very few neurologists on the ground, lack of a standardized theater to manage neurosurgical cases, lack of neuro-specialized nurses, neurophysiotherapists, and neurophysiology tools, inadequately equipped ambulances, and improper neurointensive care and neuromonitoring modalities [10]. As it stands, referral of such cases remains the best option. However, in our setting, the option to transfer the patient to a better health facility also remains challenging due to factors such as long transport distances (estimated 2–9 h away) [10] by ground ambulance depending on the distance to the nearest referral hospital, which in a nutshell, could make the patient lose the golden hour for the management of such conditions, as these patients are either unstable from the get-go or deteriorate rapidly from a stable state within minutes to a few hours.
4 Conclusion
Cerebellar hemorrhagic CVA, acute hydrocephalus, and SAH are individually life-threatening, each registering countless complications and deaths. The simultaneous occurrence of cerebellar hemorrhagic CVA, acute hydrocephalus, and SAH is therefore a rare sight to behold, being extremely challenging to manage, especially in low-resource settings due to the lack of proper neurosurgical infrastructure and other neurosurgical resources. The complexity of these cases involves several variables to be taken into consideration, such as the size of the hematoma, the initial level of consciousness, the degree of compression of the fourth ventricle, and the presence of hydrocephalus, which are of prime importance in the prognosis and management. Furthermore, the existing controversies surrounding the management of such cases as well as the lack of consensus regarding the existing guidelines contribute to the magnanimous challenge of managing such cases. They delay decision-making and jeopardize the chances of survival of the patient, which are usually already slim. With all these caveats, the chances of survival of these patients remain meager from the onset. There is therefore the need not only for the establishment of proper neurosurgical services in our setting but also for methods to identify the appropriate initial treatment before further irreversible neurological damage and, finally, standardized guidelines for the management of these cases.
This case illustrates the fatal outcome of a patient with right cerebellar hemorrhagic CVA associated with a non-traumatic subarachnoid hemorrhage and complicated by a diffuse cerebral edema, acute obstructive tri-ventricular hydrocephalus due to compression of the fourth ventricle, and on a background of a grade III hypertension.
Author Contributions
William Ntchompbopughu Tih: conceptualization, supervision, writing – original draft, writing – review and editing. Egbe Gift Etuka: investigation, resources.
Acknowledgments
We express our gratitude to the patient and his family, who kindly consented for his case to be reported.
Consent
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




