Diagnosis and Management of Cushing's Syndrome During Pregnancy: Two Cases Report and Literature Review
Funding: The authors received no specific funding for this work.
ABSTRACT
Cushing's syndrome (CS) during pregnancy is a rare condition, with only a few reported cases in the literature. Diagnosis is often difficult due to physiological changes during pregnancy. Careful clinical observation during pregnancy and after delivery is necessary to initiate appropriate treatment.
1 Introduction
Cushing's syndrome (CS), also referred to as hypercortisolism, is a clinical disorder caused by excessive exposure to exogenous or endogenous glucocorticoid hormones. The excess cortisol level may lead to menstrual irregularities and infertility in women, making the diagnosis of CS during pregnancy relatively rare [1]. Common manifestations of CS include moon face, buffalo hump, central obesity, acne, striae, hypertension, and diabetes [2]. The clinical presentation of CS in pregnancy is complex and nonspecific. Symptoms, such as obesity, striae, hypertension, and impaired glucose tolerance, are frequently misattributed to normal pregnancy-related changes. Therefore, diagnosing CS during pregnancy is highly challenging. Pregnancy-associated CS can lead to severe maternal and fetal complications, including hypertension, preeclampsia, diabetes, fetal growth restriction, intrauterine fetal death, and preterm birth. The management of CS during pregnancy lacks evidence-based guidelines, and clinical experience is limited [3].
This study reports two cases of CS during pregnancy, aiming to explore their clinical features, diagnostic approaches, and maternal–fetal outcomes, providing insights for the treatment and management of pregnancy-associated CS.
2 Case Presentation
2.1 Case 1
2.1.1 Case History/Examination
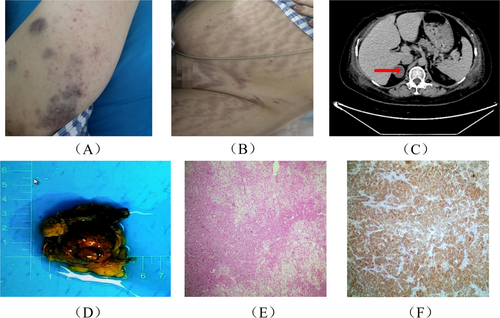
A 28-year-old female patient, G2P1, at 40 weeks of gestation, was diagnosed with an intrauterine pregnancy. During pregnancy, she developed personality changes, auditory and visual hallucinations, insomnia, fatigue, hyperglycemia, and hypertension. Her weight increased by 25 kg during pregnancy. Due to fetal distress, she was admitted to our hospital and delivered a live female infant who presented with cyanosis, weak respiration, hypotonia, a heart rate of 60 bpm, and no crying. The infant was promptly transferred to the neonatal intensive care unit for resuscitation. Postpartum, the patient experienced approximately 300 mL of blood loss. Due to severe hypokalemia, respiratory distress, and oxygen desaturation, she was transferred to the ICU. Although her condition improved, hypokalemia recurred, and her blood pressure remained poorly controlled. She had two previous pregnancies with no miscarriages. Upon admission, the patient's physical examination revealed a temperature of 36.6°C, a heart rate of 102 beats per minute, a respiratory rate of 20 breaths per minute, a blood pressure of 152/100 mmHg, a height of 162 cm, a weight of 85 kg, a waist circumference of 105.0 cm, and a body mass index (BMI) of 32.38 kg/m2. Notable findings included a moon-shaped face, multiple vascular lesions, buffalo hump, central obesity, a fatty pad on the back of the neck, increased body hair on the limbs, and scattered blue-purple bruising, predominantly on the limbs. The skin appeared thin, and cardiovascular and pulmonary examinations were unremarkable. Longitudinal purple striae were observed on the abdomen, hips, and thighs (Figure 1A,B).
2.1.2 Methods (Differential Diagnosis, Investigations, and Treatment)
Laboratory results (summarized in Table 1) indicate a significant reduction in adrenocorticotropic hormone (ACTH) levels, while serum cortisol (COR) and 24-h urinary free cortisol (UFC) levels were significantly elevated. An abdominal-enhanced CT scan revealed a right adrenal adenoma, small amounts of ascites, and edema of the chest and abdominal walls (Figure 1C). Pituitary MRI showed no significant abnormalities. Pathological examination of the right adrenal mass, which measured 5 × 7 × 3 cm, revealed a 3.8 × 3.2 × 2 cm tumor completely encapsulated, consistent with an adrenal cortical adenoma (Figure 1D). There was local invasion, but the capsule remained intact. Hematoxylin and eosin (HE) staining suggested tumor tissue infiltration characteristic of malignancy (Figure 1E). Immunohistochemical testing was positive for synaptophysin (Syn) (Figure 1F), indicating neuroendocrine differentiation. This suggests the possibility of an ectopic ACTH-secreting neuroendocrine tumor or an adrenal tumor with neuroendocrine features.
| Laboratory examination | Case 1 | Case 2 | Reference value and range |
|---|---|---|---|
| White blood cells (×109/L) | 13.13 | 12.88 | 3.69–9.16 |
| NE (%) | 76.7 | 83.8 | 50–70 |
| ALT (IU/L) | 719.0 | 8.4 | 0–40 |
| AST (IU/L) | 323.2 | 12.6 | 0–40 |
| Blood glucose (mmol/L) | 2.59 | 3.06 | 3.5–5.5 |
| FT3 (pmol/L) | 1.51 | 2.47 | 1.71–3.71 |
| FT4 (pmol/L) | 0.78 | 9.08 | 0.70–1.48 |
| TSH (μIU/mL) | 0.15 | 1.01 | 0.27–4.20 |
| ACTH (pg/mL) 8:00 | 1.60 | 1.00 | 7.2–63.6 |
| ACTH (pg/mL) 16:00 | 1.53 | 1.00 | 7.2–63.6 |
| ACTH (pg/mL) 0:00 | 1.00 | 1.00 | 7.2–63.6 |
| COR (μg/dL) 8:00 | 23.82 | 24.45 | 3.7–19.4 |
| COR (μg/dL) 16:00 | 23.32 | 23.79 | 3.7–19.4 |
| COR (μg/dL) 0:00 | 19.65 | 21.26 | 3.7–19.4 |
| UFC (μg/24 h) | 1224.9 | 1866.9 | 3.5–45 |
- Abbreviations: ACTH, adrenocorticotropic hormone; ALT, alanine aminotransferase; AST, aspartate aminotransferase; COR, cortisol; FT3, free triiodothyronine; FT4, free thyroxine; NE (%), neutrophil (%); TSH, thyroid stimulating hormone; UFC, urinary free cortisol.
The patient was treated with sustained-release nifedipine (20 mg, twice daily) to control blood pressure, spironolactone (20 mg, twice daily), and sustained-release potassium chloride (1 g, three times daily) to stabilize serum potassium levels (3.59–4.75 mmol/L). The patient underwent laparoscopic adrenalectomy for the excision of a right adrenal adenoma. Postoperatively, hydrocortisone (20 mg in the morning, 10 mg in the afternoon) was administered as replacement therapy for more than 2 months.
2.2 Case 2
2.2.1 Case History/Examination
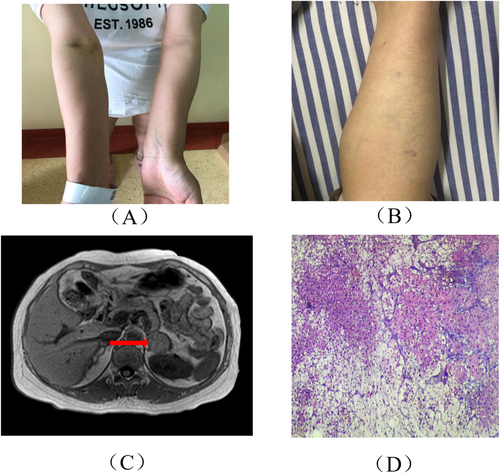
A 33-year-old female patient, G5P1, at 20 weeks of gestation, presented with irregular menstruation, with a cycle occurring twice per month and reduced menstrual flow. A pregnancy test was positive, confirming a diagnosis of an intrauterine singleton pregnancy. Additionally, the patient had central hypothyroidism and a history of gestational diabetes. She experienced five pregnancies, including one live birth, two spontaneous miscarriages, and one induced abortion. Her eldest daughter was diagnosed with congenital heart disease and underwent surgical intervention. There were no significant abnormalities in her personal or family medical history. Upon admission, physical examination revealed a temperature of 36.8°C, heart rate of 90 bpm, respiratory rate of 20 breaths per minute, blood pressure of 140/90 mmHg, height 158 cm, weight 90 kg, and BMI of 23.4 kg/m2. The patient exhibited a moon-shaped face, slight hirsutism at the temples, a small mustache on the upper lip, conjunctival edema, and erythematous patches with scales on the left axilla and posterior left thigh, with well-defined borders. Her skin appeared thin, and capillaries were visible in the lower limbs. Ecchymosis was noted on her extremities (Figure 2A,B).
2.2.2 Methods (Differential Diagnosis, Investigations, and Treatment)
Laboratory tests revealed a significant decrease in ACTH levels, with a notable increase in serum COR and 24-h UFC levels (Table 1). MRI of the pituitary and adrenal glands revealed a left adrenal adenoma, with no significant abnormalities on the pituitary scan (Figure 2C). Pathological examination of the left adrenal mass revealed an adrenal gland measuring 6 × 3 × 3 cm, with an additional 4 × 2.5 × 2.5 cm mass encased in a complete capsule, consistent with an adrenal cortical adenoma. HE staining suggested tumor tissue infiltration characteristic of malignancy (Figure 2D).
The patient was managed with insulin for glycemic control, labetalol (100 mg once daily) for blood pressure reduction, sustained-release potassium chloride (1 g three times daily) to supplement potassium and maintain serum levels between 3.5–4.3 mmol/L, and thyroid hormone replacement (50 μg once daily). The patient underwent laparoscopic left adrenalectomy for the removal of an adrenal adenoma. Postoperatively, the patient initiated hydrocortisone replacement therapy (20 mg in the morning, 10 mg in the afternoon) for a duration of 6 months.
3 Conclusion and Results (Outcome and Follow-Up)
3.1 Case 1
One month postoperatively, the patient's blood pressure was 128/92 mmHg, ACTH was 31.07 pg/mL, serum potassium was 3.74 mmol/L, and thyroid function was normal, prompting discontinuation of levothyroxine sodium (Table 2). At the one-month follow-up, blood pressure was 120/80 mmHg, COR at 8:00 AM was 11.60 μg/dL, serum potassium was 4.09 mmol/L, and hydrocortisone was discontinued (Table 2). At the 13-month follow-up, the patient's blood glucose, blood pressure, and psychological state were all normal, and her daughter's growth and development were consistent with peers.
| Laboratory examination | Case 1 | Case 2 | Reference value and range |
|---|---|---|---|
| White blood cells (×109/L) | 4.10 | 3.81 | 3.69–9.16 |
| NE (%) | 55.5 | 57.2 | 50–70 |
| ALT (IU/L) | 20.2 | 11.3 | 0–40 |
| AST (IU/L) | 28.3 | 20.2 | 0–40 |
| Blood glucose (mmol/L) | 5.18 | 4.01 | 3.5–5.5 |
| FT3 (pmol/L) | 8.17 | — | 1.71–3.71 |
| FT4 (pmol/L) | 14.93 | 11.80 | 0.70–1.48 |
| TSH (μIU/mL) | 2.67 | 1.03 | 0.27–4.20 |
| ACTH (pg/mL) 8:00 | 31.07 | 4.97 | 7.2–63.6 |
| ACTH (pg/mL) 16:00 | — | — | 7.2–63.6 |
| ACTH (pg/mL) 0:00 | — | — | 7.2–63.6 |
| COR (μg/dL) 8:00 | 11.60 | 1.08 | 3.7–19.4 |
| COR (μg/dL) 16:00 | 0.001 | — | 3.7–19.4 |
| COR (μg/dL) 0:00 | — | — | 3.7–19.4 |
| UFC (μg/24 h) | — | — | 3.5–45 |
- Abbreviations: ACTH, adrenocorticotropic hormone; ALT, alanine aminotransferase; AST, aspartate aminotransferase; COR, cortisol; FT3, free triiodothyronine; FT4, free thyroxine; NE (%), neutrophil (%); TSH, thyroid stimulating hormone; UFC, urinary free cortisol.
3.2 Case 2
The patient underwent a lower segment cesarean section due to term premature rupture of membranes and breech presentation, delivering a healthy female infant. Over 1 month postoperatively, the patient's blood pressure was 120/80 mmHg, serum potassium was 3.88 mmol/L, fasting blood glucose was 4.01 mmol/L, and thyroid function was normal (Table 2). As a result, antihypertensive medications, insulin, sustained-release potassium chloride, and levothyroxine were discontinued. Hydrocortisone was continued with a dosage of half a tablet in the morning and a quarter tablet in the afternoon.
4 Discussion
CS is a complex condition caused by prolonged overproduction of cortisol by the adrenal cortex. Hypercortisolism can directly affect the gonads and inhibit the secretion of gonadotropins from the hypothalamic–pituitary axis [4]. Female patients often present with menstrual irregularities, secondary amenorrhea, and approximately 75% experience ovulatory dysfunction. Untreated patients rarely conceive naturally [5]. Consequently, pregnancy-associated hypercortisolism is extremely rare, with approximately 200 reported cases of CS during pregnancy worldwide [6, 7]. Normal physiological changes during pregnancy can mask the symptoms of CS, making early diagnosis and treatment challenging [8]. Corticosteroid excess poses significant risks to both the mother and fetus, often leading to adverse pregnancy outcomes, such as preterm birth, preeclampsia, and intrauterine fetal death [9].
Despite clinical overlap, normal pregnancy does not typically present with the specific features of CS, such as purple striae, proximal muscle weakness, and skin thinning or bruising [10]. A minority of patients may exhibit symptoms similar to mania or schizophrenia, as seen in Case 1, where mental changes were initially attributed to typical pregnancy responses and overlooked. It is essential to exclude hypokalemia caused by vomiting during pregnancy, and frequent low potassium levels during prenatal checkups should be carefully evaluated [3]. Additionally, hypokalemia induced by corticosteroid use during pregnancy should also be considered to avoid interference with the diagnosis of CS [11]. CS is characterized by chronically elevated cortisol levels, which can result from pituitary tumors, adrenal tumors, ectopic ACTH secretion, or prolonged glucocorticoid therapy [12]. Since cortisol levels physiologically rise during pregnancy while maintaining a normal circadian rhythm, the diagnosis of CS should only be considered when salivary cortisol levels are significantly elevated, the diurnal cortisol rhythm is absent in blood tests, and urinary free cortisol exceeds three times the normal range (with sampling on different days, ≥ 2 times) [13]. Due to pregnancy-induced increases in ACTH concentrations, it is difficult to localize the source of ACTH overproduction to the pituitary or adrenal glands [14]. Therefore, when multiple ACTH tests (from different days, ≥ 2 times) show levels below 2.2 pmol/L (< 10 pg/mL), a non-ACTH-dependent form of CS is suggested, and adrenal ultrasound should be performed [15]. Given the limited sensitivity of ultrasound, if results are negative, adrenal MRI can be considered in the second or third trimester after careful evaluation of the associated risks and benefits [16].
In Case 1 and Case 2, the elevated levels of WBC and NE (%) may be primarily due to the significant increase in cortisol, which leads to an increase in total white blood cell and neutrophil counts, while promoting lymphocyte apoptosis and the redistribution of lymphocytes and eosinophils [17, 18]. In Case 1, the patient had a high BMI during pregnancy, fatty liver, and hypokalemic crisis. In addition to hypertension and psychological abnormalities, other systemic issues, such as coagulation dysfunction, were also present, resulting in a critical condition. Given the fatty liver-induced liver damage, it is highly likely that severe acute stress triggered a worsening of liver injury (evidenced by elevated ALT and AST levels). Elevated cortisol is the most significant factor affecting thyroid-stimulating hormone (TSH) secretion in CS patients. Studies have observed an inverse relationship between cortisol and TSH levels, with both endogenous and exogenous hypercortisolism inhibiting serum TSH levels [19]. Another potential mechanism for TSH suppression in CS is the effect on type 2 deiodinase activity, which converts T4 to T3 in the hypothalamus and pituitary [20]. Increased type 2 deiodinase activity induced by hypercortisolism leads to elevated local T3 levels, which ultimately result in the suppression of thyrotropin-releasing hormone (TRH) and TSH secretion.
Surgical treatment remains the most effective first-line option for CS during pregnancy [21]. For patients with adrenal adenomas, treatment may involve unilateral adrenalectomy during pregnancy or postdelivery, followed by pharmacological management. In late pregnancy, pharmacological treatment and early delivery are prioritized. After unilateral adrenalectomy, due to the increased cortisol demand during pregnancy, there may be functional insufficiency of the contralateral adrenal gland, necessitating daily corticosteroid replacement therapy [22]. Pharmacological treatments are generally not recommended for pregnancy-related CS to minimize potential teratogenic exposure and avoid fetal adrenal insufficiency [23]. However, for patients with persistent clinical manifestations or those unable to undergo surgery, steroid synthesis inhibitors, particularly metyrapone, remain the most used therapy [24].
Uncontrolled CS during pregnancy can result in a higher incidence of maternal complications. Even in treated cases, some patients may experience complications, such as gestational hypertension or preterm birth. In deciding on the appropriate treatment, factors such as the stage of pregnancy, underlying etiology, severity of hypercortisolism, and potential treatment benefits must be considered. However, for women receiving treatment during pregnancy, higher fetal survival rates have been observed. In Case 1, the patient was diagnosed with CS postpartum. After 42 days, she underwent laparoscopic adrenalectomy and was subsequently treated with hydrocortisone replacement therapy, which was gradually tapered. After approximately 1 year of follow-up, both the patient and her daughter's blood pressure, blood glucose, blood potassium levels, and thyroid function returned to normal, and skin striae significantly improved. The patient's mental symptoms also resolved, and her daughter's growth and development were consistent with those of her peers. Further differences will require evaluation in subsequent assessments. In Case 2, the patient was diagnosed with pregnancy-associated cortisol excess around 22 weeks gestation. After multidisciplinary consultation and communication with her family, she declined surgery and chose to manage blood glucose, blood pressure, and potassium levels. She delivered successfully, and neither the mother nor the child experienced severe complications.
Due to the overlap between physiological and pathological changes in pregnancy and the clinical presentation of CS, conditions such as hypertension, hypokalemia, obesity, and striae should prompt further investigation into possible hypercortisolism. Obstetricians and endocrinologists should heighten their awareness of pregnancy-associated CS, and early diagnosis and intervention can significantly improve maternal and fetal outcomes.
Author Contributions
Jingjing Huang: conceptualization, writing – original draft. Zhiyu Deng: data curation, investigation. Guohua Li: supervision. Deshui Zhao: writing – original draft, writing – review and editing.
Ethics Statement
The authors have nothing to report.
Consent
Written informed consent was obtained from the two patients to publish this report in accordance with the journal's patient consent policy.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All the data used and analyzed during this study are available from the corresponding author upon reasonable request.




