Left Ventricular Apical Thrombosis as an Early Alarming Indicator of Left Ventricular Apical Pseudoaneurysm
Funding: The authors received no specific funding for this work.
ABSTRACT
In patients with myocardial infarction, left ventricular pseudoaneurysm is uncommon and may present insidiously. Left ventricular apical thrombosis may accompany the left ventricular apical pseudoaneurysm and may be an early alarming indicator. Accordingly, close follow-up of patients with left ventricular apical thrombosis in the context of myocardial infarction via appropriate imaging modalities may be required.
1 Introduction
Left ventricular pseudoaneurysm (LVP) is a rare and fatal complication in 0.2%–0.3% of patients with ST-elevation myocardial infarction (MI). Although diagnosis is challenging according to nonspecific clinical manifestations, early diagnosis is mandatory because of the 30%–45% risk of cardiac rupture [1]. Hence, LVP should be detected and managed early, and alarming signs, symptoms, and imaging evidence should be considered. In the diagnosis of LVP and its differentiation from LV true aneurysm, imaging modalities, such as echocardiography, computed tomography, and cardiac magnetic resonance imaging (CMR), have a major role. These imaging modalities may provide early evidence for these complications [2].
2 Case History/Examination
A 63-year-old man with a history of hypertension was admitted to the emergency department with typical chest pain at rest. Physical examination was unremarkable. Electrocardiography showed sinus tachycardia and ST elevation in leads V1–V6, suggesting extensive anterior MI.
3 Methods
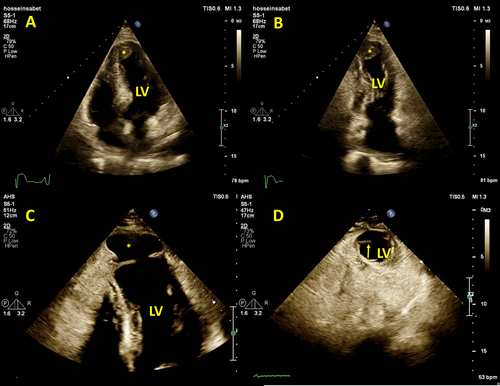
The patient underwent emergent coronary angiography, which demonstrated three-vessel disease and occlusion of the left anterior descending artery in the mid-part. He underwent a successful primary percutaneous coronary intervention with a drug-eluting stent. Two days later, transthoracic echocardiography (TTE) revealed reduced LV ejection fraction (=35%) with akinesia of all apical segments and mid-septal segments and prominent LV apical thrombosis (Figure 1A,B and Video 1).
The patient was discharged with drug treatment, including aspirin, clopidogrel, metoprolol succinate, sustained-release nitroglycerin, captopril, spironolactone, atorvastatin, and apixaban. In addition, CMR was requested for further evaluation.
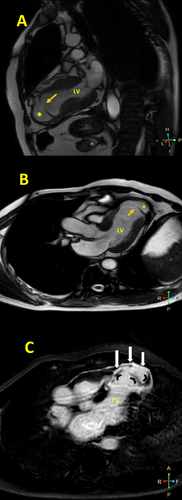
A CMR study 19 days after MI demonstrated an LV ejection fraction of 35%, significant regional wall motion abnormality in the anterior circulation, and an apical LVP (neck = 10 mm and depth = 17 mm) with no thrombosis. A first-pass perfusion study confirmed the diagnosis of pseudoaneurysm, and a late gadolinium enhancement (LGE) sequence showed transmural LGE in the mid-septal and all apical segments and the pseudoaneurysm wall, with the presence of coronary microvascular obstruction (CMVO) in the apical segments and at the site of the ruptured LV wall (Figure 2, Videos 2 and 3).
A second TTE examination, 21 days after the MI, showed findings compatible with the CMR findings. There was also to-and-fro flow through the LVP neck in a color Doppler study (Figure 1C,D and Video 4), and there was a moderately sized hyperechoic and nonhomogeneous pericardial effusion, suggestive of intrapericardial hematoma due to the ruptured LV.
Two days later, the patient underwent surgical repair, and intraoperative findings confirmed the diagnosis.
4 Conclusion
In patients with MI, LVP is an uncommon and severe complication that may present insidiously. Consequently, any alarming evidence in history, physical examinations, and imaging modalities should alert clinicians to consider LVP. The alarming evidence can be nonspecific, such as LV apical thrombosis, but may accompany the early stage evolution of LVP. Hence, frequent imaging monitoring of similar patients may be required.
5 Discussion
LVP is a rare post-MI complication as a result of ventricular free wall rupture that is limited by the pericardium, thrombosis, and hematoma with a high probability of rupture [3]. LVP usually has a narrow neck with an orifice diameter to cavity diameter ratio of < 0.5 and to-and-fro flow at the pseudoaneurysm neck as revealed by color Doppler studies. Pericardial effusion with varying echogenicity due to blood and thrombosis is another piece of evidence [4]. The most common location of LVP is the posterior wall, but it can be detected in the lateral, apical, and inferior walls. Chest pain, dyspnea, heart failure, ventricular arrhythmia, and thromboembolism are the most frequent symptoms; nonetheless, about 10% of patients are asymptomatic and incidentally diagnosed [5].
Although TTE is an appropriate initial imaging modality for LVP diagnosis, other diagnostic modalities, such as CMR, are more sensitive in detecting LVP [6].
A large and sudden tear in the LV free wall results in pericardial tamponade and cardiogenic shock [7]. The true mechanism of LVP has not been determined; nevertheless, it has been proposed that when there is a tear in a small, fragile segment of the LV wall, a small orifice connects the LV cavity to a potential space under the pericardium. Blood flows backward and forward through the orifice, and with passing time, it results in the outpouching of this area. The adherent pericardium and thrombus limit the myocardial tearing, which finally evolves into LVP [8].
Old age, female sex, hypertension, first MI, and the absence of collateral circulation are risk factors for LVP [9]. One of the predisposing factors for post-MI complications is CMVO, which can be detected by CMR using LGE sequences as dark hypo-signal areas within transmural infarcted regions. CMVO is related to the no-reflow phenomenon and is associated with an increased mortality risk and adverse remodeling [10, 11].
In our presented case, a history of hypertension without any history of previous MI and CMVO in CMR were risk factors for LVP. Although successful revascularization of the culprit coronary artery was done, the presence of CMVO points to the probable occurrence of the no-reflow phenomenon. The patient was asymptomatic after MI, implying the slow evolution of a myocardial tear to an LVP.
In our case, the first TTE revealed an LV apical thrombosis, and we prescribed apixaban for treatment. Follow-up showed the resolution of the LV apical thrombosis; however, an apical LVP was detected. It seems that the slow progression of the myocardial tear prevented the occurrence of a catastrophic event while the patient received anticoagulation. Further, the LV apical thrombosis may accompany an LVP because thrombosis in the context of MI is a marker of significant necrosis and inflammation [12]. Thus, close follow-ups of these patients via appropriate imaging modalities can be beneficial.
Author Contributions
Flora Fallah: conceptualization, data curation, resources, supervision, visualization, writing – original draft. Sahar Asl-Fallah: conceptualization, data curation, resources, supervision, writing – original draft, writing – review and editing. Mani Ghorbanzadeh-Aghdam: conceptualization, data curation, resources, supervision, visualization, writing – review and editing. Ali Hosseinsabet: conceptualization, data curation, formal analysis, supervision, validation, visualization, writing – original draft, writing – review and editing.
Consent
All authors have consented to the publication of the article. Written informed consent was obtained from the patients to publish this report in accordance with the journal's patient consent policy.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All data associated with the article are available if required.




