Necrotizing Pancreatitis in a Patient Diagnosed With SLE: A Rare Case Report
Funding: The authors received no specific funding for this work.
ABSTRACT
Necrotizing pancreatitis is an extremely rare but serious complication of systemic lupus erythematosus (SLE). Early recognition and appropriate treatment, including steroids and immunosuppressive therapy, are critical for improving outcomes in SLE patients with this condition.
1 Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that can affect various organs and tissues throughout the body. In this autoimmune disease, multiple organ systems including the skin, joints, kidneys, heart, pancreas, lungs, brain, and blood cells are involved [1].
Necrotizing pancreatitis (NP) is a severe and potentially life-threatening inflammatory condition affecting the pancreas. NP is characterized by tissue death, and can occur in individuals with various underlying conditions, and SLE is among the potential contributors. While lupus primarily affects the joints, skin, kidneys, and other organs, it can also impact the pancreas. Pancreatic involvement in SLE may present with a range of symptoms, including abdominal pain, nausea, and vomiting [2]. However, NP as a complication of SLE is exceptionally rare, with very few reported cases.
2 Case History/Examination
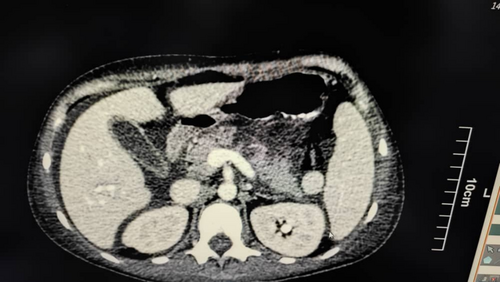
We present a case report of a 17-year-old girl with problems that started a year ago, in 2023, in the form of multiple bruises on the whole body and bleeding from the gums. She was diagnosed with immune thrombocytopenic purpura (ITP) and treated with corticosteroids and intravenous immune globulin (IVIG). The patient had no problems until 2 months ago, in September 2024, when she was hospitalized due to leukopenia and seizures. After investigations, proteinuria of 1100 mg/dL, lymphopenia, leukopenia, positive antinuclear antibody (ANA), anti-double-stranded DNA (anti-dsDNA), and reduced complement levels were detected (Table 1). During hospitalization, due to abdominal pain and swelling, and shifting dullness on examination, a CT scan of the abdomen and pelvis with oral and intravenous contrast was performed (Figure 1). NP was reported, with involvement of less than 30% of the pancreas, in addition to the presence of abundant free fluid in the abdomen and pelvis and bilateral pleural effusion.
| Test | Result | Normal range | Unit |
|---|---|---|---|
| ESR | 40/20 |
For males: 0–15 mm/h For females: 0–20 mm/h |
mm/h |
| CRP | Negative | Less than 10 mg/L | mg/L |
| F ANA | 12 | 1 | — |
| Anti dsDNA | 349 | 30 | — |
| Antiphospholipid | Negative | — | — |
| C3 | 0.03 | 90–180 | mg/dL |
| C4 | 0.4 | 10–40 | mg/dL |
| CH50 | 30 | 50–150 | mg/dL |
| BUN | 24 | 8–20 | mg/dL |
| CA | Normal | — | — |
| Ph | Normal | — | — |
| TG | Normal | — | — |
| Creatinine | 0.8 |
Adult males: 0.6–1 Adult females: 1.3 0.5–1.1 |
mg/dL |
| AST | 31 | 10–40 | U/L |
| ALT | 27 | 7–56 | U/L |
| ALKP | 144 | 44–147 | U/L |
| Amylase | Normal | 1–8 | U/L |
| Lipase | 360 U/L | 100 | U/L |
| Baseline WBC | 2500 | 4500–11,000 | cells/μL |
| Final WBC | 4660 | ||
| Baseline HB | 7.5 g/dL |
Males: 13.8–17.2 g/dL Females: 12.1–15.1 g/dL |
g/dL |
| Final HB | 11 g/dL | 80–100 | Femtoliters |
| PLT | 226,000 | 150,000–450,000 | platelets/μL |
| Baseline urine protein 24 h | 1083 mg/dL | 0–14 | mg/dL |
| Final urine protein 24 h | 566 mg/dL | 0–14 | mg/dL |
- Abbreviations: ALKP, alkaline phosphatase; ALT, alanine aminotransferase; Anti dsDNA, anti-double-stranded DNA antibodies; AST, aspartate aminotransferase; BUN, blood urea nitrogen; C3, complement component 3; C4, complement component 4; CA, calcium; CH50, complement total blood test; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; F ANA, fluorescent antinuclear antibody; HB, hemoglobin; LDH, lactate dehydrogenase; Ph, phosphors; PLT, platelet count; TG, triglyceride; WBC, white blood cell count.
Due to the seizures, MRI/MRV was performed for the patient. The MRI revealed diffuse hyperintensity at the subcortical white matter of the high frontal lobes and parieto-occipital lobes bilaterally, while the MRV was normal. To investigate other causes of pancreatitis, MRCP was performed and reported as normal. Based on the history of thrombocytopenia, leukopenia, lymphopenia, proteinuria, seizures, positive ANA, anti-dsDNA, and reduced complement levels, SLE was diagnosed. The patient was treated with methylprednisolone pulses for 3 days, followed by prednisolone at a dosage of 1 mg/kg and cyclophosphamide pulses at 500 mg. Other potential causes of pancreatitis were investigated but no pathological findings were identified. The patient's pancreatitis was attributed to SLE. During her hospitalization, she received routine treatment for pancreatitis.
Currently, during follow-up, cytopenia and ascites have improved, seizures have not recurred, and 24-h proteinuria is at 566 mg/dL. The patient is receiving her third dose of cyclophosphamide, 20 mg of prednisolone, and 200 mg of hydroxychloroquine daily.
Differential diagnosis, investigations, and treatment differential diagnoses included autoimmune pancreatitis, drug-induced pancreatitis, and idiopathic pancreatitis. Investigations such as MRCP ruled out biliary causes. The patient was treated with methylprednisolone pulses, oral prednisolone (1 mg/kg), cyclophosphamide pulses (500 mg), and hydroxychloroquine (200 mg daily). Supportive care for pancreatitis was provided, including fluid management and nutritional support.
3 Differential Diagnosis, Investigations, and Treatment
Differential diagnoses included autoimmune pancreatitis, drug-induced pancreatitis, and idiopathic pancreatitis. Investigations such as MRCP ruled out biliary causes. The patient was treated with methylprednisolone pulses, oral prednisolone (1 mg/kg), cyclophosphamide pulses (500 mg), and hydroxychloroquine (200 mg daily). Supportive care for pancreatitis was provided, including fluid management and nutritional support.
4 Conclusion and Results (Outcome and Follow-Up)
The patient's condition improved significantly after treatment, with reduced proteinuria (566 mg/dL), resolution of cytopenia and ascites, and no recurrence of seizures. She remains on maintenance therapy, including the third dose of cyclophosphamide, prednisolone, and hydroxychloroquine, with regular follow-up.
5 Discussion
In this case report, we reported a 17-year-old person whose constellation of her symptoms raises the suspicion of both SLE and NP. Pancreatitis is a medical condition characterized by the inflammation of the pancreas; Symptoms of pancreatitis typically involve severe abdominal pain.
Pancreatitis should primarily be classified into two broad categories: acute pancreatitis and chronic pancreatitis. Acute pancreatitis can further be subdivided based on clinical and morphological criteria into interstitial edematous pancreatitis and NP. The latter represents a severe progression involving is necrosis of pancreatic and, potentially, peripancreatic tissue. Chronic pancreatitis is a distinct and prolonged inflammatory condition of the pancreas, marked by irreversible morphological changes and often persistent impairment of function.
NP, a severe form of acute pancreatitis characterized by the necrosis of pancreatic tissue, is less common than acute pancreatitis. Studies generally report that NP occurs in 5%–10% of all acute pancreatitis cases. The incidence of acute pancreatitis in SLE patients is itself relatively rare; therefore, the subset developing NP would understandably be smaller and less frequently reported. Also, the diagnosis of NP often involves advanced imaging techniques such as contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI). Patients with SLE are frequently managed with concern for exposure to contrast media due to potential nephrotoxicity, especially in those with lupus nephritis. This could lead to underdiagnosis or delayed diagnosis of NP in this population.
AP, as a result of SLE is more common rather than NP [3]. While AP manifests more frequently than NP in patients with SLE, timely medical intervention in the acute inflammatory stage may attenuate progression to pancreatic necrosis in this population [4]. SLE can affect small blood vessels, resulting in inflammation known as vasculitis [5]. When this inflammation occurs in the blood vessels supplying the pancreas, it can restrict blood flow to the organ. This impaired perfusion deprives the pancreatic tissue of oxygen and nutrients. The ischemia and resulting dysfunction may then trigger the onset of AP in susceptible individuals [6]. Beyond the pancreas, vasculitis damage from SLE can impact organs throughout the body. This can also predispose patients to NP through ischemia caused by vascular inflammation.
The pathophysiological underpinnings of SLE and pancreatitis encompass intricate molecular, immunological, and genetic interactions [7]. SLE has a substantial genetic component, which engenders immune perturbation aberrant B and T lymphocyte reactivity, cytokine disproportion, and epigenomic alterations critically regulate disease propagation. The hallmark interferon-alpha signaling cascade overload characterizes SLE immunopathology.
In comparison, pancreatitis molecular factors such as genetic controllers of pancreatic enzymes, inflammatory mediators, and autoantibodies (notably in autoimmune pancreatitis) are operant. Premature pancreatic digestive enzyme activation, oxidative injury, and genetic liability collectively fuel pancreatitis' development.
Despite differing specifics, SLE and pancreatitis share an immunoregulatory breakdown and inflammation as a cardinal unifier. Both conditions exhibit the loss of self-tolerance, enabling autoimmune injury and tissue damage [8]. Comprehending the molecular intricacies is vital to further enable targeted therapeutics and superior handling of these compound autoimmune and inflammatory illnesses. Recent studies have been shown that patients with SLE often have higher rates of metabolic conditions like diabetes and hypertriglyceridemia [9]. These are also independent risk factors for developing pancreatitis. Also, cytokine imbalances due to overactive immune systems in SLE may promote inflammation in various organs, including theoretically the pancreas.
While numerous studies and reports extensively cover AP, there is a scarcity of literature and research focusing on NP due to its rarity.
To the best of our knowledge, although there are several case reports of lupus-associated AP, only one case of lupus-associated NP has been reported. In July 2014, a 37-year-old woman of African descent, previously diagnosed with SLE, presented with sudden abdominal pain, accompanied by nausea and vomiting. Despite being under ongoing treatment with mycophenolate mofetil (MMF), prednisolone, hydroxychloroquine (HCQ), and belimumab, she encountered recurrent episodes of AP. Diagnostic assessments, including laboratory tests and imaging, verified the presence of pancreatitis with 20% necrosis. The medical team suspected SLE-induced pancreatitis, prompting the administration of high-dose steroids. This intervention yielded favorable responses in both clinical symptoms and biochemical markers. Following this, the patient's ongoing care plan incorporated rituximab for enhanced disease management. A subsequent follow-up examination indicated notable improvement in the pancreatitis condition. This case highlights the complexities of addressing complications associated with SLE and underscores the effectiveness of tailored approaches, including high-dose steroids and rituximab, in managing AP and achieving positive patient outcomes [10].
Also, retrospective studies indicate that 0.9% to over 5% of SLE patients suffer from AP. Among 264 SLE patients studied, predominantly female with an average age of 31.4 years, abdominal pain was a consistent symptom. AP was more common in those with shorter disease duration, high activity scores, and multiorgan involvement. Diagnosis as “idiopathic” SLE-related AP was based on guidelines, excluding other causes. Managing SLE-related AP is challenging due to the lack of a standardized approach, and the use of glucocorticoids is debated due to potential complications. Monitoring serum lipase levels after high-dose steroids is suggested. Some studies suggest a positive outcome with plasma exchange, and the occurrence of AP in SLE may signal an association with macrophage activation syndrome, with reported mortality rates of up to one-third of cases [11].
6 Conclusion
In conclusion, our article presented a female with SLE, developed NP, which is a rare complication of SLE. This case underscores the importance of early recognition and intervention to improve patient outcomes.
Author Contributions
Maryam Javid: conceptualization, investigation, methodology, resources, writing – original draft. Tohid Damideh: data curation, validation, visualization. Omid Pourbagherian: funding acquisition, software, writing – original draft, writing – review and editing. Mehdi Jafarpour: project administration, supervision, validation.
Acknowledgments
The authors express their gratitude for the valuable contribution made by the patient in providing blood donation. The authors appreciate support provided by Imam Reza Hospital, Tabriz, Iran.
Consent
Written informed consent was obtained from the patient to publish this case report in accordance with the journal's patient consent policy.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request. Due to the rarity of the condition and privacy concerns, the data are not publicly available.




