A Rare Presentation of Fahr's Syndrome Associated With Secondary Hyperparathyroidism
Funding: The authors received no specific funding for this work.
ABSTRACT
Fahr's syndrome (FS) is a rare disorder characterized by intracerebral calcification, presenting with various neuropsychiatric symptoms. This case highlights a rare presentation of FS with secondary hyperparathyroidism. It underscores the importance of comprehensive evaluation of early symptoms, effective use of diagnostic procedures, and proper management of symptoms.
1 Introduction
Fahr's syndrome (FS) is a rare neurodegenerative disorder with a prevalence of less than 1 case/million, characterized by abnormal intracerebral calcifications, particularly in areas controlling movements [1, 2]. FS is named after Karl Theodor Fahr, a German neurologist who first described it [3]. FS presents with various neuropsychiatric symptoms, including seizures, movement disorders, extrapyramidal syndromes, depression, dementia, and hallucinations [4]. Diagnosis is based on the presence of progressive neurological symptoms and the identification of calcified deposits in the brain through imaging assessments. It is crucial to exclude other potential causes, as FS can often be misdiagnosed due to its resemblance to different conditions [1]. Various factors can contribute to the development of FS, such as endocrine disorders, particularly disturbances in parathyroid function [5, 6]. These conditions include hypoparathyroidism, pseudohypoparathyroidism, pseudo-pseudohypoparathyroidism, and hyperparathyroidism [1]. While hypoparathyroidism is the most common, hyperparathyroidism is rarely reported [7, 8].
Despite the rarity of FS, this case report presents an infrequently reported instance of diagnosing and managing FS associated with secondary hyperparathyroidism (SHPT).
2 Case History/Examination
A 32-year-old woman was admitted to the acute emergency ward of a tertiary care facility with complaints of dizziness, repeated episodes of vomiting that began on the morning of admission day, and recent fatigue. Upon admission, she showed a loosening of associations, incoherent speech, and stereotyped behaviors. Her parents reported lifelong difficulties with concentration and learning from her teenage years, alongside a progressive decline in cognitive function that had become significant over the past year.
The patient's past medical history revealed seizures, which had not occurred in the past 10 years, and kidney disease, likely glomerulonephritis, during childhood, although the exact details were unclear to the family. Additionally, her creatinine level was 1.36 approximately 1 year ago. Both conditions were not followed up seriously due to the family's low socioeconomic status. At the time of admission, the patient was not on any medication, and there was no family history of similar cases, including FS.
The patient's level of consciousness was normal, with a Glasgow Coma Scale score of 15/15. Cognitive impairment was primarily assessed using the Mini-Cog, which scored 1 out of 5, and the Mini-Mental State Examination, which yielded 13 out of 30. Additionally, the Montreal Cognitive Assessment (MoCA) scored 11 out of 30 for further evaluation, with the most significant impairments observed in executive functions and visuospatial skills. Her vital signs were normal, except for a heart rate of 116 bpm. On neurological examination, the patient reported no pain or numbness. There was no evidence of spasticity or sensory abnormalities, but proximal limb weakness and ataxia were noted.
3 Methods
At this stage, given the wide range of signs and symptoms, various differential diagnoses were considered, including neurodegenerative disorders like Parkinson's disease and metabolic disorders such as hypokalemia or hypocalcemia. Further paraclinical evaluations were necessary to rule out all other potential causes.
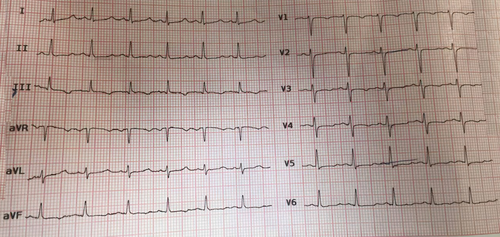
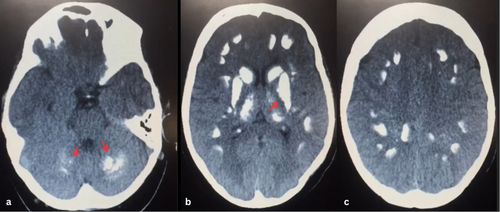
Diagnostic electrocardiography (ECG), revealed a prolonged QT interval (Figure 1), indicating a probable electrolyte imbalance. A non-contrast spiral computed tomography (CT) scan of the brain was requested due to neuropsychological symptoms. The scan revealed extensive cerebral calcifications (Figure 2), most frequently in the lenticular nucleus, particularly the internal globus pallidus. The calcifications also extended to the putamen, thalamus, caudate, and dentate nuclei, with predominant calcifications in regions outside the basal ganglia.
Laboratory investigations revealed hypocalcemia, with a serum calcium level of 5.8 mg/dL, and hyperphosphatemia, with a serum phosphate level of 7.4 mg/dL. Further testing showed a vitamin D level of 13 ng/mL and an elevated parathyroid hormone (PTH) level of 181 pg/mL. Blood urea nitrogen was 19.6 mg/dL, and creatinine was 1.66 mg/dL. Urinalysis indicated proteinuria and hematuria. Other laboratory parameters, including complete blood count, erythrocyte sedimentation rate, C-reactive protein, albumin, blood glucose, blood electrolytes, serum magnesium, iron, ferritin, venous blood gas, and thyroid hormones, were all within normal ranges (Table 1).
| Parameters | Result | Reference range |
|---|---|---|
| Blood glucose | 117 mg/dL | 70–140 mg/dL |
| Hemoglobin | 12.5 g/dL | 12.1–15.1 g/dL (Women) |
| WBCs | 6300/μL | 4000–11,000/μL |
| Platelets | 185,000/μL | 150,000–450,000/μL |
| BUN | 19.6 mg/dL | 7–20 mg/dL |
| Creatinine | 1.66 mg/dL | 0.6–1.2 mg/dL |
| TSH | 2 mIU/L | 0.4–4.0 mIU/L |
| Parathyroid hormone | 181 pg/mL | 10–65 pg/mL |
| Vitamin D | 13 ng/mL | 20–50 ng/mL |
| Albumin | 3.9 g/dL | 3.4–5.4 g/dL |
| Serum phosphate | 7.4 mg/dL | 2.6–4.5 mg/dL |
| Serum sodium | 136 mmol/L | 135–145 mmol/L |
| Serum potassium | 3.6 mmol/L | 3.5–5.0 mmol/L |
| Serum calcium | 5.8 mg/dL | 8.6–10.3 mg/dL |
| Serum magnesium | 1.9 mg/dL | 1.7–2.2 mg/dL |
| Serum iron | 88 mcg/dL | 50–170 mcg/dL (Women) |
| Ferritin | 138 | 24–307 ng/mL (Women) |
| Venous pH | 7.37 | 7.35–7.45 |
| Venous pCO2 | 41 mmHg | 35–45 mmHg |
| Venous HCO3 | 23 mmol/L | 22–26 mmol/L |
| ESR | 9 mm/h | 0–20 mm/h (Women) |
| CRP | 4 mg/L | Less than 10 mg/L |
Based on symmetrical and bilateral brain calcifications (Figure 2), progressive neuropsychological symptoms, childhood glomerulonephritis, and parathyroid dysfunctions, a diagnosis of FS associated with secondary SHPT was made.
A slow intravenous infusion of calcium gluconate was administered, with serum levels checked every 2 h until the calcium level reached 10.2 mg/dL. During hospitalization, the patient received daily doses of haloperidol and calcium gluconate, along with vitamin D3.
4 Outcome and Follow-Up
The patient was hospitalized for 3 days and at the time of discharge, dizziness, weakness, and ataxia persisted, but the severity of these symptoms significantly decreased, and vital signs were normal. Calcium level was reported as 9.8 mg/dL, the ECG showed no abnormalities, and the MoCA score had improved to 18 out of 30. Treatment was switched to oral administration of calcium carbonate, vitamin D3, and cinacalcet, along with daily doses of risperidone and clonazepam while restricting dietary phosphorus. The patient was referred to a psychiatrist for cognitive disorder management and to an endocrinologist. One-year follow-up of the patient revealed no recurrence of seizures. Routine checks of electrolytes and vitamin D showed no abnormalities, but cognitive impairment persisted.
5 Discussion
FS and Fahr's disease are two distinct conditions, both characterized by idiopathic brain calcifications associated with a diverse range of clinical presentations, particularly neurological and psychiatric symptoms [7, 9]. While Fahr's disease is considered the primary form genetically linked either as an autosomal dominant or recessive trait manifesting between the ages of 40 and 60, FS represents the secondary form and is associated with underlying causes such as endocrinopathies, infections, mitochondrial myopathy, celiac disease, and toxic agents. It affects both sexes equally and typically appears earlier, between the ages of 30 and 40 [7, 10, 11].
Psychiatric symptoms are found in 45% of cases, varying from mild concentration and memory problems to personality or behavioral changes, and can include severe conditions like psychosis and dementia [1, 12]. A decline in cognitive performance is observed in most patients with FS, with a mean MoCA score of 24.6 out of 30. The cognitive domain most often impaired is executive functioning, affecting 42% of patients [13]. In our case, the MoCA score of 11, with significant impairments in visuospatial skills and executive functions, shows lower cognitive function than typically seen in FS patients. FS primarily manifests as movement disorders in approximately 55% of patients. Some patients may present with neurological symptoms like dystonia, ataxia, chorea, and extrapyramidal syndromes [3, 14]. Neurological symptoms, often understated compared to the extent of anatomic and radiological lesions, are common. It can be seizures, pyramidal syndrome, akinetic hypertonic syndrome, cerebellar dysfunctions, urinary problems, choreoathetosis movements, dysarthria, or mumbling speech [7]. Parkinsonism can also be observed in FS, but brain atrophy and degeneration are typically seen in CT scans of Parkinson's disease [15, 16]. This excludes Parkinson's disease from the differential diagnosis.
Due to the absence of precise criteria and the broad spectrum of symptoms, further investigations, including laboratory and imaging studies, are essential to prevent misdiagnosis [1, 11]. CT scans are crucial for diagnosing FS, as they demonstrate the location of cerebral calcifications more clearly than MRI. However, MRI is useful for detecting metabolic processes that have not yet progressed to full calcification [1, 3, 17]. The lenticular nucleus and internal globus pallidus are most affected by these calcifications. Calcifications are commonly found in the putamen, thalamus, caudate, dentate nuclei, cerebral cortex, cerebellum, centrum semiovale, brain stem, subcortical white matter, and hippocampus. Occasionally, calcifications are more prominent in areas outside the basal ganglia. A symmetrical pattern of calcifications in the basal ganglia strongly supports the diagnosis of FS [1, 18].
Calcification in the brain tissue associated with FS is thought to arise from disruptions in phosphorus and calcium levels, coupled with alterations in the blood–brain barrier's integrity [19]. Pathological studies reveal that calcium deposits are typically found in the extracellular or extravascular space, particularly surrounding small blood vessels [20]. Elevated activity of alkaline phosphatase in the brain, particularly within the basal ganglia, despite normal blood levels, may lead to the deposition of calcium phosphate in nervous tissue [3]. SHPT is an adaptive and sometimes maladaptive process associated with CKD. It is characterized by elevated PTH levels, reduced synthesis of active vitamin D, and hypocalcemia caused by hyperphosphatemia [21, 22]. Hyperphosphatemia, with levels ranging from 7 to 9 mg/dL, can cause calcium precipitation in soft tissues such as the brain. However, the exact mechanism by which hyperphosphatemia affects the concentration of free calcium ions in tissues remains unclear. While metabolic and inflammatory diseases can initiate calcification, genetic factors play an important role in its occurrence [23, 24]. These irreversible calcifications, primarily composed of calcium phosphate and calcium carbonate, may also contain gluconate, mucopolysaccharides, iron, copper, magnesium, zinc, aluminum, silver, and cobalt [1, 19]. Additionally, the prolonged QT interval observed in the ECG is caused by hypocalcemia, which impairs myocardial contractility and can lead to ventricular arrhythmias [21, 25].
FS with an endocrine origin is more commonly associated with hypoparathyroidism or pseudohypoparathyroidism, and its association with hyperparathyroidism is notably infrequent [7]. FS associated with SHPT is exceedingly rare and has not been extensively investigated.
Currently, there is no definitive treatment for FS. Disease management involves symptomatic treatment to improve outcomes, but the prognosis remains unpredictable [3, 26, 27]. An important point in the management of FS patients with a history of seizures is to consider platelet count due to the risk of intracranial hemorrhage [28]. In our case, the platelet count was measured at 185,000 (Table 1), which assured us of a reduced risk of hemorrhage.
Correction of calcium and phosphate levels, along with treatment using vitamin D3, should be considered in association with parathyroid disorders [1]. Additionally, the management of SHPT involves restricting phosphorus intake and administering cinacalcet, along with calcium-based phosphate binders such as calcium carbonate [29-31]. Intravenous calcium solutions, including 10% calcium gluconate or 10% calcium chloride, are part of the management of severe hypocalcemia in endocrine disorders [32]. For treatment-resistant SHPT, parathyroidectomy can be effective in alleviating symptoms [33]. The response to Haloperidol in reducing neuropsychiatric symptoms during the acute phase is remarkable, but long-term management with atypical antipsychotics like Risperidone, Olanzapine, and Aripiprazole alongside Clonazepam is recommended [5, 27, 34]. Mood stabilizers, antiepileptics, and dopamine agonists have been reported as effective and could be part of the treatment plan [3, 27].
This case report highlights the complexity of diagnosing FS due to its wide range of symptoms, lack of specific diagnostic criteria, and significant management challenges, particularly when associated with endocrine disorders. The case of a young woman diagnosed with FS in the context of SHPT is exceedingly rare. The lack of proper follow-up for chronic conditions since childhood has played a significant role in its progression. A comprehensive approach, integrating neuropsychiatric and metabolic symptoms, along with attention to paraclinical findings, was crucial in reaching the final diagnosis in this case. As a learning point, FS should be considered as a differential diagnosis in SHPT if associated with neuropsychiatric symptoms.
Author Contributions
Simin Najafgholian: conceptualization, investigation. Mahbod Soltani: project administration, writing – original draft. Sanaz Amirian: investigation, methodology. Negar Pourahmadian: validation, writing – review and editing.
Acknowledgments
We would like to express our sincere gratitude to Mr. Mohammad Satarzadeh for his valuable help in data curation for this case report. His dedication and support were instrumental in the completion of this study.
Disclosure
The authors have nothing to report.
Consent
The legal representative of the patient, due to the patient's cognitive impairment, provided written informed consent for the publication of this case following the journal's patient consent policy. Patient information was obtained from hospital archives, ensuring privacy was maintained throughout.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data from our article will be available upon reasonable request.