Improved Motor Function in Cervical Spinal Cord Injury Following Spinal Cord Stimulation
Funding: The authors received no specific funding for this work.
ABSTRACT
Spinal cord stimulation (SCS) is a promising therapy for the management of spinal cord injury. Following SCS, this patient experienced a significant reduction in pain and decreased spasticity, which ultimately led to improved upper extremity function.
1 Introduction
Spinal cord injury (SCI) is a serious medical condition that leads to significant morbidity and mortality. In the United States alone, there are approximately 300,000 people living with SCI, and about 17,000 new cases are reported each year with a cost of almost $10 billion annually [1]. Approximately two-thirds of SCIs are incomplete injuries, where some neural activity still remains below the lesion, thus preserving partial function. Unfortunately, studies have reported that even in patients with incomplete SCI, there is a substantial decrease in quality of life following their injury, along with a significant reduction in life expectancy [2, 3].
The outcome of a SCI varies depending on the location and degree of neurological damage. Some complications, such as the motor spasticity seen in our patient, affects 65%–92% of people with chronic SCI and is more common with higher levels of injuries [3-5]. Spasticity is a velocity-dependent increase in muscle tone associated with upper motor neuron (UMN) injuries [6]. The damaged UMN leads to a loss of inhibitory signals in the descending spinal tracts, resulting in increased stretch reflex and muscle tone [7]. Spasticity typically begins to develop several weeks or months after injury as the period of areflexia begins to subside and can lead to pain, discomfort, and complications with significant functional impairment, further contributing to decreased quality of life for patients [2, 6, 8].
Epidural spinal cord stimulation (SCS) is a neuromodulation technique that places electrodes within the epidural space of the dorsal column to deliver mild electrical impulses. Although SCS has traditionally been employed as a treatment for chronic pain, recent research suggests its potential usefulness for other medical conditions as well. In this case report, we hope to add to this potential by presenting a patient who had a long-standing history of pain and spasticity as a result of SCI and found impactful improvements with the use of SCS.
2 Case History
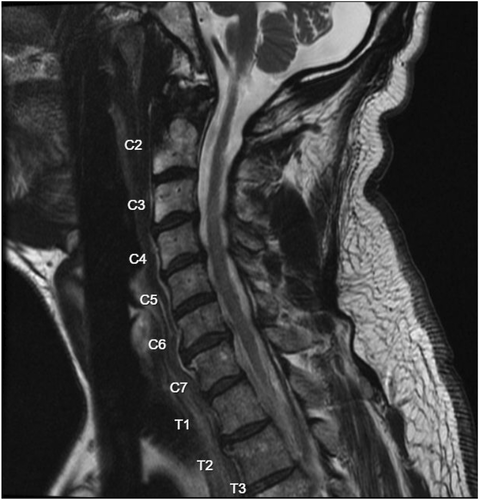
A 60-year-old male with a history of traumatic cervical SCI secondary to a ski accident presented to the pain management clinic with chronic spasticity and neuropathic pain of the right upper extremity (RUE) and bilateral lower extremities (BLE) (Figure 1). The patient had previously found minimal relief with pharmacologic management, physical rehabilitation, or chemodenervation with botulinum toxin.
Physical examination demonstrated increased tone in his RUE, resulting in loss of function. His Modified Ashworth Score (MAS), a tool used to grade spasticity severity, was graded as a 3/5 throughout his RUE indicating marked tone throughout range of motion. He had residual weakness in his right upper and lower extremities, with significant deficiencies in wrist and hand strength (2/5) and bilateral hip flexor strength (4/5). He had moderate shoulder, biceps, and triceps strength (4/5). His right arm was prone and stiff due to spasticity, making supination difficult and his fingers unable to extend. Reflexes were brisk (3+) across various muscles including biceps, brachioradialis, triceps, patellar, hamstrings, and gastrocnemius. He also had complaints of paresthesia and numbness in his BLE. After thorough testing according to the International Standards for Neurological Classification of SCI (ISNCSCI), the patient's injury was classified as C5 AIS D.
3 Methods
The patient was agreeable to trying other treatment modalities and subsequently underwent a SCS trial, with cervical and thoracic leads placed to cover mid-C3-C5 and mid-T9-T11, respectively. This trial revealed a significant improvement in the patient's right-sided neuropathic pain, demonstrating a greater than 70% reduction in symptoms. This positive outcome supported the continuation of the current treatment strategy, leading to the scheduled implantation of a SCS.
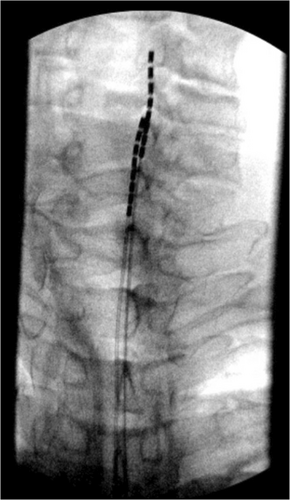
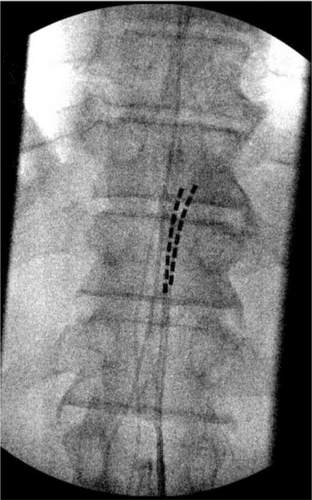
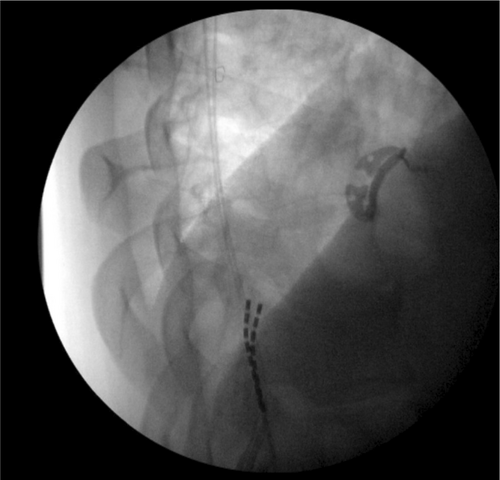
During the SCS implant, two 8-contact spinal cord stimulator leads were inserted and advanced under AP and lateral fluoroscopic guidance into the posterior epidural space. The left lead was advanced to the level of C5, and the right lead to the level of C4 (Figure 2). The thoracic leads were placed midline at the level of the T11 vertebral body (Figure 3). The placement of the leads within the posterior epidural space was confirmed with lateral fluoroscopy (Figure 4). Intraoperative paresthesia stimulation confirmed adequate coverage of the pain areas, and the leads were anchored and battery placed in a typical fashion. The patient was awake and conversant throughout the procedure.
4 Conclusion and Results
Two weeks post-procedure, the patient reported improvement in his RUE spasticity and neuropathic pain. Neuropathic pain in the feet was mostly masked by the stimulator. However, he was still able to notice it and stimulator settings were adjusted for better coverage. Subsequent weeks involved several programming adjustments to optimize pain management and spasticity reduction. These adjustments led to the restoration of the patient's spastically impaired right elbow extensor motor function, achieved with a frequency of 2 Hz, a pulse width of 550 ms, and an intensity of 5 amps.
The patient reported a 60% improvement in spasticity and a 50% improvement in mobility of his RUE. There was also marked improvement of spasticity on physical exam, with a MAS grading of 1+/5 for elbow extension, signifying only a slight increase in muscle tone compared to prior examinations. The patient noted that he had been finally able to gain enough function back to start elbow extension exercises with weights, a feat he once found impossible. To go along with this improved function, he also reported complete alleviation of pain, rating it at 0 out of 10. This allowed for discontinuation of all analgesic medications.
The patient approved the reporting of this case and results.
5 Discussion
SCI can have devastating consequences for patients, affecting their physical, psychological and social well-being [9]. Common impairments associated with SCI are loss of motor and sensory function, bowel and bladder dysfunction, recurrent infections, autonomic dysreflexia, spasticity, contractures, and chronic pain. These impairments can also impose a significant financial burden on patients, who may incur costs ranging in the millions over their lifetime for SCI-related care [10]. The main goal of SCI treatment is to help patients enhance their functional abilities, mitigate pain, and ultimately cope with their condition in the best way possible.
Current treatment strategies for post-SCI spasticity involve multimodal strategies and a multidisciplinary approach. Initial treatment is typically conservative with passive muscle stretching and physical therapy, pharmacologic agents (tizanidine and benzodiazepines), onabotulinumtoxinA injections, or even surgical interventions (e.g., dorsal rhizotomy). Baclofen can additionally be administered through an intrathecal baclofen pump (ITB), providing sustained bolus release of the medication for spasticity management. While these treatment options are widely used, they come with limitations, including undesirable adverse effects, treatment resistance, and inconsistent results.
Technological advances have allowed for the development of minimally invasive techniques that can advance patient care and expand available treatment options. In 1967, by way of the “Gate Control Theory,” it was postulated that the introduction of an exogenous electrical signal can potentially modulate the endogenous pain signals that coalesce within the dorsal column. Even though the exact underlying process of how SCS enhances functional recovery remains somewhat unclear at this time, the prevailing hypothesis is that the constant stimulation of afferent fibers in the dorsal root elevates the overall excitability of spinal circuits, making interneurons and motor neurons closer to their firing threshold and physiological state [11]. These effects enable the neurons to respond more effectively to the diminished inputs after an injury, thus increasing synaptic strength and plasticity. Although the mechanism is not fully understood, there is growing evidence that the suppression of motor neuron hyperactivity can also improve spasticity in patients with SCI [12]. Ongoing pathophysiologic research is needed to explain the underlying mechanism, but select patients are already experiencing tangible benefits from this treatment option.
SCS for improving spasticity after SCI is an emerging technology showing positive results overall [13, 14]. Although SCS has traditionally been employed as a treatment for chronic pain, recent research suggests its potential usefulness for other medical conditions as well. A 2022 systematic review found that SCS could be beneficial in restoring sensorimotor function, including volitional movement, after SCI. The total participants included 327 patients with SCI, and of the studies assessing sensorimotor function, 71 out of 127 (56%) patients regained volitional movement during SCS [15]. In addition, a 2024 systematic review analyzed 34 studies for spasticity improvements with the use of SCS. A subset of their data looked specifically at subjective improvement in spasticity after SCI, where 190 out of 281 (68%) patients found improvement in their symptoms after SCS [13]. Our case study adds to this existing data, showing that neuromodulation holds promise as a tool that may enhance patients' functional recovery in conditions beyond pain, such as spasticity from SCI.
Standard SCS parameters for SCI typically include a frequency of 40–100 Hz, a pulse width of 300–600 μs, and an amplitude of 3.6–8.5 mA for conventional stimulation [16]. These parameters are chosen to optimize neural activation and therapeutic effects, balancing effective pain relief, and motor function. Our patient showed improvement with a mix of theses parameters. Of note, he showed optimal function with a frequency of 2 Hz, which is not typically standard for SCI treatment. However there is evidence supporting the efficacy of low frequencies in certain contexts. For instance, Hofstoetter et al. demonstrated that 2 Hz stimulation can evoke unmodulated reflexes in the lower limbs of individuals with motor-complete SCI, suggesting some potential for neuromodulation [17]. Moreover, Hentall et al. found that very low pulse rates, including 2 Hz, were effective in reducing chronic pain in SCI patients, indicating that low-frequency stimulation can have therapeutic benefits [18].
Our case report highlights the efficacy of these specific stimulation parameters in managing spasticity and reducing pain, offering starting parameter guidance for future patient treatments. Along with these parameters, this case is notable for its rare application of multi-site lead placements in SCS, targeting both cervical (C3-C5) and thoracic (T9-T11) levels, ensuring comprehensive symptom coverage. This approach led to a significant reduction of over 70% in right-sided neuropathic pain during the trial phase and complete pain alleviation post-implantation, allowing discontinuation of all analgesic medications. Compared to typical SCI pain management outcomes, this represents a substantial improvement, emphasizing the effectiveness of multi-site lead placement and SCS in chronic neuropathic pain management without pharmacological intervention.
While there is emerging evidence for the potential role of SCS as a treatment modality for UMN lesion-induced spasticity, more research is needed to assess efficacy, optimal stimulation parameters, and proper patient selection. SCS presents a few risks, including infection, hematoma, fibrosis, and lead migration, that should also help guide further exploration. With the advent of new technologies that can measure neural feedback during stimulation and the supraspinal/cortical changes occurring in addition to analgesia, there may be a lot more to be uncovered with future investigations.
This case report illustrates the remarkable benefits of SCS for a patient with an incomplete AIS D SCI. After receiving SCS, he reported significant reductions in RUE spasticity and neuropathic pain, as well as enhanced motor function and range of motion due to these improvements. We hope that this case study helps provide further evidence that spinal cord stimulators have the potential to positively impact the lives of patients with SCI.
Author Contributions
Michael Suarez: writing – original draft, writing – review and editing. David M. Gallacher: writing – original draft, writing – review and editing. David S. Jevotovsky: project administration, supervision, writing – review and editing. Harman Chopra: conceptualization, investigation, supervision, visualization, writing – review and editing. Mustafa Broachwala: resources, supervision, writing – review and editing. Joel P. Castellanos: conceptualization, data curation, funding acquisition, investigation, project administration, resources, supervision, validation, writing – review and editing.
Consent
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




