Acute graft-versus-host disease and bronchiolitis obliterans after autologous stem cell transplantation in a patient with multiple myeloma
Key Clinical Message
Sixty-seven-year-old patient, diagnosed with multiple myeloma who had received autologous stem cell transplantation, following bortezomib, dexamethasone and thalidomide conventional regimen, achieving complete response, developed rash, diarrhea, and severe respiratory failure, 80 days after the transplantation procedure. He was diagnosed with graft-versus-host disease and bronchiolitis obliterans syndrome.
Introduction
Multiple myeloma (MM) is the second most frequent hematologic neoplastic disease after non-Hodgkin lymphoma. The median age at the moment of diagnosis is over 65 years 1. Incidence rates of MM in the European population are estimated to be around 3, eight cases per 100,000 inhabitants and year 2, 3.
The International Myeloma Working Group consensus has just updated the disease definition of multiple myeloma to include validated biomarkers in addition to existing requirements of attributable CRAB features (hypercalcemia, renal failure, anemia, and bone lesions). These changes are based on the identification of biomarkers associated with near inevitable development of CRAB features in patients who would otherwise be regarded as having smoldering multiple myeloma (more than one focal lesion at magnetic resonance Imaging (MRI), plasma cell bone marrow infiltration superior to 60% or involved vs. uninvolved free light chain ratio higher than 100) 4. In symptomatic MM patients, treatment is initiated in order to achieve rapid and high-quality responses. Different strategies are considered depending on the age and performance status of our patients and the eligibility to receive autologous stem cell transplantation (ASCT) or not.
Most patients newly diagnosed with MM are older than 65 years and nontransplant eligible. In this group of patients, the introduction of new treatment options has changed the aims of therapy, and complete response (CR) is now also considered as a main goal. Alkylator-containing regimens are available and melphalan plus prednisone (MP) has been the backbone to which novel agents were added resulting in new standards of care. The addition of bortezomib or thalidomide to MP (VMP) showed a significant benefit in terms of PFS and OS 5. Bendamustine in combination with prednisone (BP) is also available in Europe. Currently, nonalkylator regimens emerged due to the positive results obtained with lenalidomide plus low-dose dexamethasone as continuous therapy in comparison with the previous standard MPT and it is a new standard of care. However, elderly patients include a very heterogeneous patients' population and physicians should consider the patient's biological age and comorbidities as well as to evaluate the degree of functional impairment in order to select the most appropriate drug regimen and adapt its dose, and optimize the supportive care treatment with bisphosphonates, antibiotics, antiviral therapy, anticoagulants, growth factors, and pain control 6.
Patients younger than 65 are considered eligible to ASCT, and the main goal is also to achieve complete durable remission and of the highest quality as possible because it is a surrogate marker for progression-free survival and overall survival. Three-drug combinations are usually used as induction, including proteasome inhibitor and dexamethasone and the third drug can be cyclophosphamide, adriamycin, or immunomodulatory drug 7, 8. After 3–6 induction cycles, high-dose chemotherapy followed by autologous stem cells transplantation (HDT-ASCT) is the standard of care at the present time 6, 9, 10. Induction therapy followed by HDT-ASCT are complementary strategies and up to 50% of patients achieve complete response. Posttransplant strategies, such as consolidation and maintenance represent attractive approaches in order to improve or maintain the quality of the response resulting into a significant prolongation of the duration of response.
Although the role of HDT-ASCT in the upfront setting is under debate, it continues being the standard of care. Its benefits are it is a nonexpensive procedure, and it has no significant impact in the quality of life. In addition, it is known that patients who relapse after ASCT are usually sensitive to rescue therapy with novel agents 12-16.
Transplant-related mortality is very low and it does not exceed 2% 16 and the toxicity is usually derived from the administration of high-dose chemotherapy and less frequently from the infusion of the cryopreserved hematopoietic stem cells. The development of some unexpected and rare transplant-related complications, such as graft-versus-host disease (GVHD), increases the mortality rate and must be known 17.
Recently, it has been described the development of acute GVHD in patients that undergo ASCT and it has been estimated a 4–13% incidence of GVHD post-HDT-ASCT 17-19. Among MM patients, the incidence is slightly higher than what has been observed in patients with other underlying diseases, such as non-Hodgkin lymphoma 18, 19. This could be explained, at least in part, because most young and fit newly diagnosed MM patients receive HDT-ASCT as part of the upfront treatment; however, specific MM therapies such as proteasome inhibitors have been implicated in the pathogenesis of this entity.
Hypotheses that explain GVHD development after autologous transplantation postulate a loss of peripheral and central lymphocyte regulation that allows the performance of CD8+ autoreactive effector cells; these, enhanced by CD4+ 20, lead to cell death. NK cells may also participate in this process 21. It has also been described that CD8+ cells recognize invariant chain fragment peptides (CLIP), which are associated with major histocompatibility complex (MHC) 22. This loss of immune regulation has been attributed to several reasons, such as the potential contribution of proteasome inhibitors hampering the removal of cellular debris which may be presented as auto antigens to cytotoxic T lymphocytes 23. However, we have also to consider that bortezomib is a reversible proteasome inhibitor that, after performance, sets the proteasome free in <72 h after administration 24.
Deregulation of T lymphocytes (particularly T regulatory cells) may be altered because of induction chemotherapy 25. Other previously described causes for GVHD syndromes in autologous recipients are: GVHD due to microchimerism of fetal cells (observed in women) and transfusion associated GVHD 26.
Recently, it has been described an association between lymphocytes subpopulations and several HLA with the development of acute GVHD in recipients of ASCT. Apparently, lower percentages of CD3+, CD8+, and HLA-B55 expression may be predisposing characteristics to develop this disease 27.
Bronchiolitis obliterans syndrome (BOS) has been associated with the transplant procedure, and usually results in the development of chronic GVHD; its pathophysiology, however, has not been well elucidated. In autologous transplant, data are limited to few cases histologically confirmed and it has been related with poor prognosis in adults 28-30. Although histological diagnosis is essential for definitive assessment of bronchiolitis obliterans disease and bronchiolar pathology, clinical approach, supported by image and functional tests can establish a suspicion diagnosis of BOS 28-30.
Here, we present the case of a 67-year-old male diagnosed with multiple myeloma who developed both GVHD and BOS postautologous transplant.
Case Report
Sixty-seven-year-old male patient diagnosed, in November 2012, with IgG kappa multiple myeloma. He presented 4, 14 translocation and plasmocytoma as poor prognostic factors. The patient received five induction cycles of bortezomib as proteasome inhibitor, thalidomide as immunomodulatory drug and dexamethasone (VTD), reaching stringent CR after fourth cycle. Peripheral blood stem cell collection was performed and he subsequently received melphalan 200 mg/m2 followed by autologous stem cell transplantation (HDT-ASCT). Patient did not present any severe transplant-related acute complication and the engraftment occurred at day +12 for granulocytes and at day +11 for platelets.
On day +84 after HDT-ASCT, he was admitted at the hospitalization unit presenting fever, with trunk and limb rash, cough, expectoration, dyspnea, and watery diarrhea. The suspect was infection in an immunocompromised and nonneutropenic patient after HDT-ASCT. Empiric antibiotic therapy and topical corticosteroids on skin lesions were started.
The laboratory findings and microbiology showed neutrophilia and elevated C-reactive protein (CRP) (value of 5.3 mg/dL with an upper edge of 0.5 mg/dL). Blood cultures were positive for Enterococcus faecium, Escherichia coli, and antibiotic therapy was expanded adding meropenem and linezolid.
Chest X-ray showed interstitial infiltrates in lower lobes. Seven days later, due to persistence of symptoms, high-resolution computerized tomography scan (HRCT) was performed evidencing reticular opacities associated with traction bronchiectasis. In spite of the high spectrum antibiotic therapy, respiratory symptoms worsened, with partial respiratory insufficiency. Pulmonary function tests (PFTs) were performed, and a mixed restrictive and obstructive pattern was observed. Bronchoscopy confirmed bronchiectasis and mucorrhea. Diagnosis of suspect was systemic infection with respiratory focus as the most probable origin of infection.
There was not an adequate clinical response to antibiotic therapy. Pulmonary function tests were repeated and they continued showing a pattern of moderate airflow obstruction (60%), with moderate restrictive defect. This results, showed no changes from the results obtained in the previous study, performed at the beginning of the episode. Both studies obtained after transplantation had dramatically changed when compared to the study performed 3 months ago, as part of the pretransplant evaluation, which was normal.
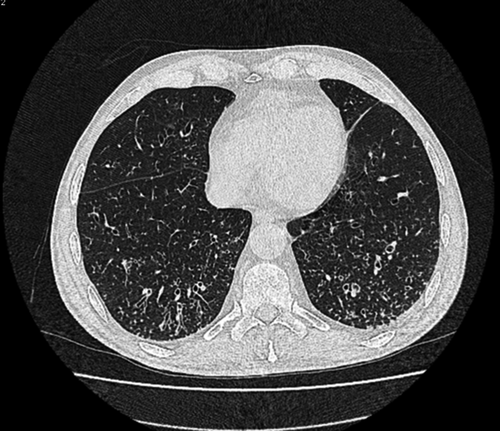
A new HRCT was requested which showed multiple bilateral centrilobular nodular opacities in the lower lobes, with tree in bud appearance, consistent with Bronchiolitis image findings (Fig. 1). These tests led to the diagnosis of BOS.
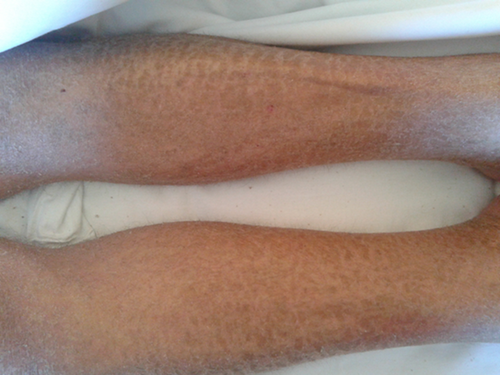
Skin lesions changed into more chronic lesions, associating more pruritus. Initial skin biopsy (interpreted as unspecific toxic/infectious pattern) was repeated, showing spongiosis, parakeratosis, and interface injury images, with vacuolated damage and apoptotic bodies. Lymphocytic perivascular infiltrates were observed, and these findings drove to the diagnosis of acute GVHD (grade II) with concomitant signs of chronicity (Fig. 2).
Due to the persistence of diarrhea, anorexia, and hypoalbuminemia, endoscopic study was done. Antral mucosa was erythematous with severe edema. Histologically, severe acute gastritis was described, consistent with GVHD.
These results led to the diagnosis of skin and gastrointestinal GVHD post-ASCT.
Graft-versus-host disease first-line therapy consisting of corticosteroids was started. Topical steroid was given both orally (for gastrointestinal manifestations) as well as applied on skin lesions (Fig. 3). For respiratory BOS, we started systemic steroid at dose of 1 mg/kg of prednisone as well as bronchodilators (b2-agonists).
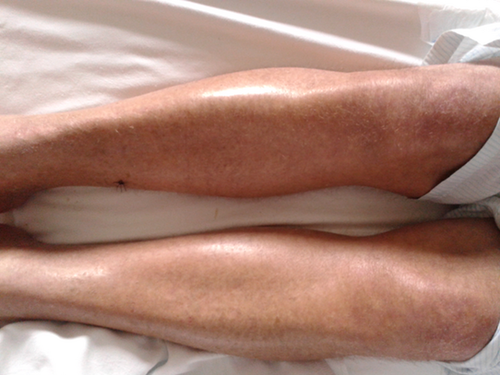
The outcome was favorable, disappearing respiratory failure and symptomatology as well. Skin and gastrointestinal manifestations also improved. After completing 15 days of full-dose steroid therapy, it was tapered and finally stopped with complete remission of symptoms.
Myeloma was evaluated and stringent complete response was maintained.
Discussion and Conclusion
Multiple myeloma is a malignant monoclonal plasma cell disorder classically characterized by osteolytic bone lesions, anemia, hypercalcemia, and renal failure 1. The International Myeloma Working Group consensus has just updated the disease definition of multiple myeloma to include validated biomarkers, in addition to existing requirements of attributable CRAB features, which are based on the identification of near inevitable development of CRAB features in patients who would otherwise be regarded as having smoldering multiple myeloma. These new criteria are as follows: more than one focal lesion at magnetic resonance imaging (MRI), plasma cell bone marrow infiltration superior to 60% or involved vs. uninvolved free light chain ratio higher than 100 4.
Patients diagnosed with multiple myeloma who are <65 years old and eligible to ASCT undergo induction therapy with a combination of three drugs, normally VTD, followed by high doses of melphalan and ASCT 10-15.
New chemotherapeutic agents (bortezomib, thalidomide, and lenalidomide) are incorporated into the initial treatment of MM, and survival with chemotherapy is improving. Whether their use will delay, reconsider, or eliminate the need for ASCT in patients with MM is not known at this time. Until the results of trials become available, upfront ASCT should remain as the standard of care for young, newly diagnosed MM patients 6, 15, 16. Also, relapses after ASCT are usually sensitive to rescue with novel agents, but we do not know about the sensitivity of plasma cells to ASCT after long exposure to novel agent-based combinations 6. It is also important to note that ASCT is not expensive and is associated with a good quality of life and very low transplant-related mortality 16. Although side effects are normally derived from high doses of therapy and less frequently from the infusion of the cryopreserved hematopoietic stem cells, rare transplant-related complications, such as graft-versus-host disease (GVHD) increase the mortality rate and must be known 17.
GVHD must be considered when making the differential diagnosis of exanthematous and diarrhea syndromes that occur in the autologous posttransplantation period. It has been estimated a 4–13% incidence of GVHD post-HDT-ASCT 17-19. Among MM patients, the incidence is slightly higher than what is observed in other diseases and maybe this is because most young and fit newly diagnosed MM patients receive HDT-ASCT as part of the upfront treatment 18, 19.
Differential diagnosis should include infections, toxic causes, enteropathy, or engraftment syndrome. In our patient, the first three-ones were not considered because of the lack of response to treatment and due to the histological findings. Peri-engraftment syndrome is an early complication that takes place around engraftment. In this case, the complication emerged in the fourth month after transplantation.
Transfusion GVHD was discarded, since our patient had received irradiated components.
Bronchiolitis obliterans syndrome (BOS) has been associated with the transplant procedure. In autologous transplant, there are limited cases histologically confirmed and related to this procedure. In adults, the rare cases reported had a poor prognosis 28-30. Because histological confirmation can rarely be obtained, BOS following HSCT is usually a clinical diagnosis that is made based upon clinical symptoms, PFT results, and radiologic findings. Respiratory infections and BOOP should be ruled out with imaging and other appropriate procedures. Airflow obstruction is the hallmark of BOS. In high-resolution computed tomography (HRCT) or thin-section CT BOS include air trapping, bronchiectasis, and bronchial wall thickening 30.
Also, BOS appears usually after day +100. In our case, clinical symptoms, HRCT images, and functional tests, modified after transplant led to the diagnosis 27, 28.
Our study reinforces the definition of acute graft-versus-host disease in autologous transplantation recipients, and as far as we are concerned, we report here the first case in which obliterans bronchiolitis is presented at the same time that aGVHD in the setting of autologous transplantation, showing complete recovery when prompt therapy was started.
Conflict of Interest
None declared.




