Cutaneous lymphocyte antigen expression loss and PD1 positivity in early cutaneous lesions of rapidly progressive mycosis fungoides
Key Clinical Message
It's important to assess cases both clinically and pathologically for factors potentially predictive of an aggressive clinical course. We concluded that the relative immunosuppressive effects of PD1 may contribute to tumor progression while the lack of staining for cutaneous lymphocyte antigen may be an additional factor facilitating distant extracutaneous migration.
Introduction
Classic mycosis fungoides (MF) or the Alibert–Bazin syndrome comprises about 72% of cutaneous T-cell lymphomas (CTCL) 1, 2. Clinically there is progression through patch, plaque, and tumor stages over a protracted period of years and oftentimes decades. The incidence of MF is higher among African Americans, in whom there is generally a worse prognosis 3. While it is considered a disease of middle-aged men 4 there is an early-onset variant that is particularly common in young African American women and may also portend a poor prognosis. Compared to other CTCLs, MF has a very favorable clinical outcome and is considered as a form of low-grade T-cell lymphoproliferative disease. Nevertheless, there are patients in whom progressive disease that involves lymph nodes, blood, and visceral organs may occur 5.
Histologically, MF shows an epidermotropic infiltrate of atypical lymphocytes with cerebriform nuclei that is usually CD4+/CD30−; there are less common variants including those, which are CD8 positive, CD4−/CD8−, and express CD56. The clinical progression of MF often coincides with histologic large cell transformation and may be associated with tumor stage MF and extracutaneous dissemination 6. The expression of chemokine receptor CXCR3 and cutaneous lymphocyte antigen (CLA) have been shown to be expressed in the neoplastic cells of MF and has been postulated to play a role in the inherent tropism of MF to the skin 7, 9. Not surprisingly, CXCR3 and CLA expressions have been shown to be lost in more advanced cases of MF 7, 8. One might postulate that the variable expression of these markers involved in lymphocyte recruitment might provide a useful tool in predicting clinical outcome in biopsy specimens from early disease.
We present a case of aggressive MF in an adult African American male, with rapid progression from patch/plaque stage to tumor stage and concurrent subsequent extracutaneous dissemination over a period of 12 months. The histologic and phenotypic predictors of this unusually aggressive presentation at the inception of his disease are discussed.
Case Report
The patient was a 47 year-old African American man with a 2-year history of CTCL. His initial presentation to an outside dermatology service was of an 8-month history of an intensely pruritic faintly erythematous, scaly patches involving the trunk, raising diagnostic consideration of atopic dermatitis or psoriasis. A skin biopsy performed in 2011 was confirmatory of MF.
He was treated with 10 sessions of narrow-band UVB phototherapy, which resulted in significant improvement of skin lesions. However, shortly following light therapy, the patient developed palpable axillary and inguinal lymphadenopathy, which lead to a biopsy of a lymph node in the later part of 2011. Subsequent to a diagnosis of nodal involvement by MF, he underwent 6 cycles of CHOP chemotherapy (cyclophosphamide, daunorubicin, vincristine, and prednisone). During chemotherapy, the appearance of more nodules led to a third biopsy, which was confirmatory of tumor stage MF. Despite treatment, the skin lesions progressed in a striking fashion, increasing in size and distribution; many of the lesions exhibited a protuberant nodular morphology. One month prior to presenting at our institution, he completed 3 cycles of romidepsin, with no improvement in skin lesions and worsening of fatigue.
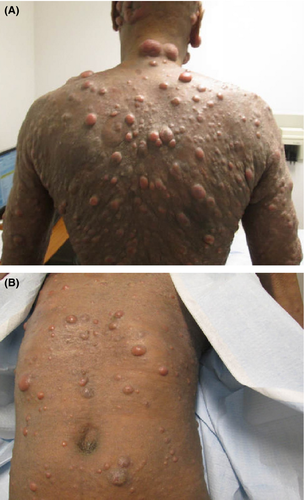
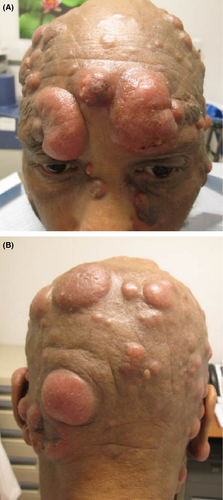
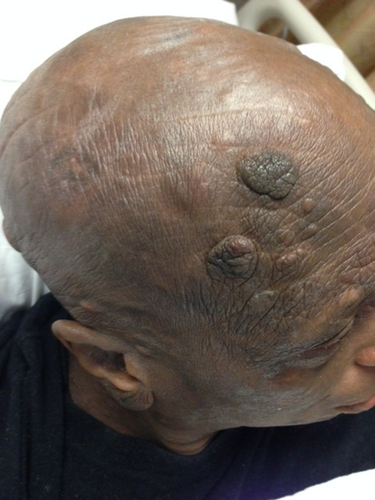
At the time of presentation to our institution in July of 2013, the patient had many systemic complaints, which included severe fatigue, loss of appetite, diffuse itching, and occasional chills. Physical exam was significant for diffuse erythroderma and scaly, and nontender erythematous nodules widespread across the face and head, neck, trunk, and extremities (Figs. 1A and B). Numerous nodules with overlying ulcerations and odorous exudates were appreciated (Figs. 2A and B). Lymphadenopathy was palpated in the submandibular, axillary, and inguinal regions. Repeat skin biopsies in July of 2013 showed tumor stage MF with large cell transformation. Radiographic imaging with PET/CT confirmed progression of disease with demonstration of hyper metabolic lesions in the neck, axillary, and femoral lymph nodes. The patient completed 4 cycles of DICE chemotherapy (dexamethasone, ifosfamide, cisplatin, and etoposide) and brentuximab vedotin (a CD30 antibody-drug conjugate) 10. The patient achieved complete resolution of the skin lesions and marked improvement in his symptoms (Fig. 3). A posttreatment PET/CT revealed complete resolution of the hyper metabolic activity and marked decrease in size of skin lesions and previously involved lymph nodes. The patient went on to receive a haplo/cord allogeneic stem cell transplantation 11. The transplant course was complicated by a central line–related acute venous thrombosis of the right internal jugular vein, corynebacterium striatum bacteremia, clostridum difficile colitis, and cord engraftment syndrome 12. Neutrophils and platelets were engrafted on day +12 post transplantation. Day +28 chimerism was consistent with a 100% umbilical cord chimerism. At the time of writing this manuscript, the patient is 240 days post transplantation, fully engrafted and has no evidence of graft versus host disease, viral reactivation, or infections.
Light microscopic findings
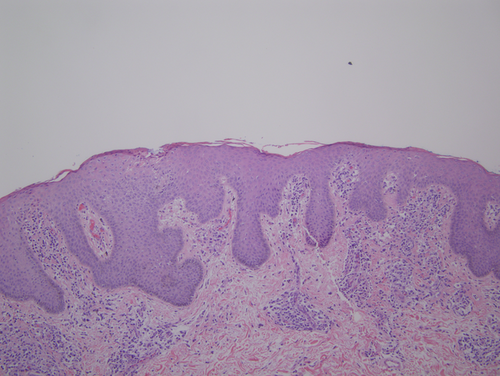
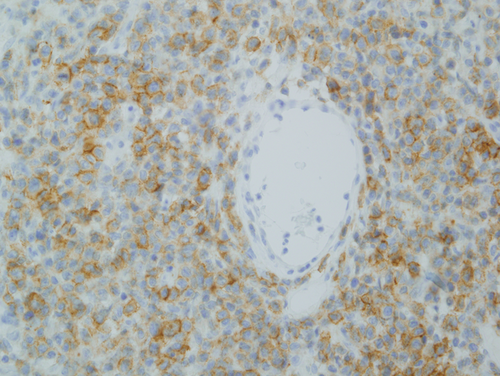
A biopsy of left lateral neck from March of 2011 showed a hyperplastic psoriasiform pattern of epidermal hyperplasia. In addition, within the dermis, there were nodular aggregates of neoplastic lymphocytes surrounding the superficial cutaneous vasculature. The abnormal lymphoid cells were in the 9–15 micron size range; however there were superficial collections of larger cells although without architectural features which would denote tumor stage MF. Some degree of epidermotropism was noted; it was focal with the main localization of lymphocytic infiltration being in the superficial dermis (i.e., nonepidermotropic) (Figs. 4 and 5). In the 20 micron size range; they exhibited irregular hyperconvoluted gyrate nuclear contours and marked hyperchromasia, typical for the cerebriform lymphocyte encountered in MF and Sezary Syndrome (SS) (Fig. 6). The neoplastic cells were positive for CD2 (Fig. 7A), CD3, CD5, CD4, and BF1. There was a striking reduction in the expression of CD7 (Fig. 7B). CD30 was positive in scattered neoplastic cells. Significant immunoreactivity was not identified for CD8, CD56, TIA-1, and granzyme. Very distinctive from a phenotypic perspective was the absence of any expression of CLA (Fig. 7C) accompanied by extensive immunoreactivity of the neoplastic cells for PD1 (Fig. 7D). Molecular studies showed a monoclonal rearrangement.
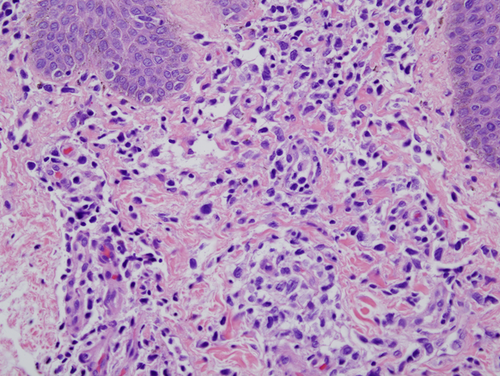
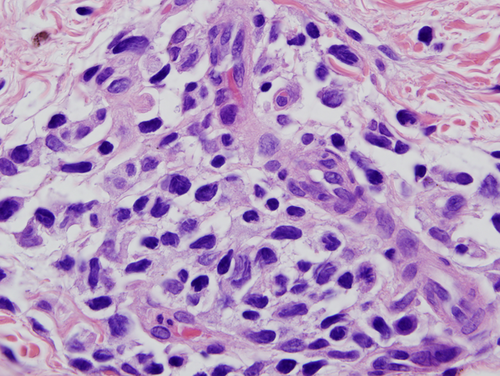
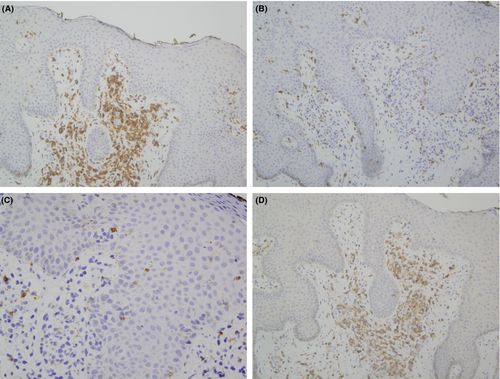
Subsequent biopsies in February of 2013 and July of 2013 appeared similar showing a massive effacing diffuse and nodular malignant lymphocytic infiltrate (Fig. 8A). The cells were in the 20–30 micron size range exhibiting a vesicular chromatin with basophilic to eosinophilic nucleoli (Fig. 8B). There were a number of atypical mitoses. A few scattered eosinophils and histiocytes were observed. While the dominant cell populace was a pleomorphic large cell, there were small foci of epidermotropism of smaller hyperchromatic and hyperconvoluted lymphocytes diagnostic of those encountered in MF. The B specimen showed the same fundamental process of a diffuse and nodular malignant large cell infiltrate. These cells were in the 15–20 micron size range; however, there was a smattering of hyperconvoluted cerebriform lymphocytes disposed amidst this malignant large cell infiltrate. The tumor cells were extensively positive for the pan T-cell markers CD3 and CD5 and were of the BetaF1 subset. There was a marked reduction in the expression of CD7. The CD30 preparation showed only a few positive-staining cells. The CD4+ and CD25+ regulatory T-cell component was very minimal and did not occupy more than 10–15% of the infiltrate; there were broad sheets of malignant cells without regulatory T-cell activity. There was significant staining for CD25. 30% to 40% of the infiltrate showed strong cytoplasmic membrane pattern of staining. As with the earlier biopsies, there was marked expression of PD-1 (Fig. 9) and a loss of immunoreactivity of the neoplastic cells for CLA (Fig. 8C).
Bone marrow biopsy and aspirate
Bone marrow biopsy and aspirate smear performed in July of 2011 showed adequate cellularity without evidence of lymphoma. A repeat bone marrow biopsy performed in October of 2013 demonstrated a similar picture.
Peripheral blood
In the peripheral blood a monoclonal T-cell rearrangement identical to that noted on his skin biopsies in July 2013 was identified i.e. In the peripheral blood a monoclonal T cell rearrangement identical to that noted on his skin biopsies in July 2013 was identified. Flow cytometry identified a small population of T cells (5%) exhibiting expression of CD57 and CD8 with loss of CD7.
Lymph node
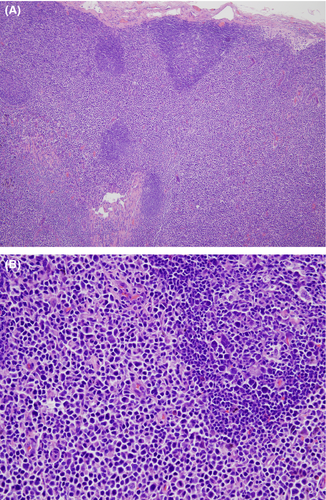
A left inguinal lymph node biopsy performed in October of 2011 demonstrated a normal architecture largely effaced by a diffuse paracortical proliferation composed of small, medium-sized to large cells (Figs. 10A and B). Residual reactive-appearing lymphoid follicles are seen. The neoplastic cells demonstrated a hyperconvoluted cerebriform appearance, similar to those in the skinbiopsy. The findings were characteristic for MF involving the lymph node.
Discussion
We present a patient with an unusually aggressive variant of MF. The time course that elapsed between the initial diagnosis of MF with clinical features of patch and plaque stage MF to one of disseminated extracutaneous lymph node disease was 8 months. There were aspects of the histopathology on the initial early plaque stage biopsy that we believe predicted this aggressive clinical course. In particular, the dominant angiocentric disposition of the superficial infiltrate, the presence of many large transformed neoplastic T cells and the absence of CLA expression were all features unusual in the morphologic realm of early MF and instead more commonly seen with advanced disease. In a 2-year period from initial diagnosis and 3 years from first becoming symptomatic with a skin rash, the patient rapidly progressed from patch/plaque stage to tumor stage MF with large cell transformation and disseminated nodal disease 7.
There is limited literature precedent regarding aggressive variants of MF. Oftentimes the clinical presentation and or histomorphologic findings are unusual. In this regard, aggressive variants have been described in the setting of bullous, vesicular, lichenoid, folliculotropic syringotropic, and granulomatous variants of MF. An unusual presentation such as one exhibiting a hemangiomatous morphology would also be an important clue clinically in regards to a more aggressive clinical course. One study focused on histomorphologic clues that could potentially predict an aggressive clinical course. The authors did not disclose features that could be considered very specific as the morphologic differences between the two groups appeared to be minimal and largely not specific. In our case report, the initial biopsy of a plaque lesion showing a superficial angiocentric infiltrate containing large cells with only focal epidermotropic in concert with marked expression of PD1 and loss in the expression of CLA, potentially defining critical clues predicting this aggressive clinical course. The histomorphology in fact was reminiscent of the changes encountered in SS which shows a similar pattern of superficial angiocentric infiltration (personal observations) and PD1 expression 13-17. All of these points will be alluded to presently.
In early stages of MF, malignant clonal T cells are found exclusively in the skin, with a significant number of these cells expressing the skin-homing receptor CLA 18. This partly explains the affinity of these cells for skin. In more advanced stages of MF, the expression of CLA is lost as shown in a study by Magro and Dyrsen 8. They showed that CLA is not expressed in either the epidermis or the dermis in cases of tumor stage MF. In contrast, earlier stage lesions of MF specifically in regards to epidermotropic patch and plaque stage MF demonstrate extensive CLA expression ranging from 70% to 100%. In contrast, diminished expression of CLA is observed in those peripheral T cell lymphomas secondarily involving the skin and as well in the more aggressive primary cutaneous lymphomas of the skin the staining pattern is distinct and separate from the extensive and uniform pattern of cytoplasmic staining encountered in MF, a point which will be alluded to presently. The correlation of the lack of CLA staining with an aggressive clinical course can be understood by recognizing the role of CLA in a normal physiologic state. In particular, organ-selective homing of lymphocytes, which primarily reside in tonsils, Peyer's patches and peripheral lymph nodes involve an interplay between receptors expressed on homing lymphocytes and their ligands present on high endothelial venules (HEV). These molecules include SELECTIN: L-, E-, and P-SELECTIN. The major lymphocyte ligand for E-selection, expressed on vascular endothelium is CLA 19. CLA plays a role in the interaction of memory T-cells and inflamed HEV 18. CLA is found in areas of chronic cutaneous inflammation and expresses a subset of circulating memory T cells 18, 20, 21. It is worth noting that CLA-expression alone does not always equate with an indolent course. For example, primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma and NK T cell lymphoma have a very aggressive clinical course with potential for extracutaneous dissemination but exhibit CLA staining 8. The staining pattern, however, in these aggressive variants of cutaneous T cell lymphoma differ from the conventional diffuse cytoplasmic staining pattern seen in MF, being that of a perinuclear dot-like pattern of positivity.
In addition, PD1 is a member of the rimmunoglobulin superfamily and plays an essential role in immune responses 22. PD1 has been identified in circulating neoplastic cells of patients with SS and in lymphoma cells of angioimmunoblastic T cell lymphoma 23, 24. The expression of PD-1 in lesions of MF is variable and the results between authors from different studies are incongruous. For example, it has been shown in one study that there is enhanced expression of PD1 on peripheral blood lymphocytes that are CD4+CD26− in patients with SS but only in those patients with a low-to-intermediate tumor burden. In patients with a high tumor burden this pattern of enhanced PD-1 expression was not seen. In healthy controls and MF patients, the peripheral blood lymphocytes fail to express PD-1. The study did not examine directly the expression of PD1 in tissue samples. They concluded that PD1 expression was a function of T-cell receptor activation and once the disease became very advanced the authors postulated that the ability for the neoplastic cells to express PD-1 in the setting of T-cell receptor activation was markedly diminished 23. In a current study, greater than 50% PD1 expression was shown in 89% of SS cases compared with only 13% of MF cases 25. The study by Wada and coworkers found significant positivity in lesional cells of MF and SS. The manner in which the study was executed was interesting. They designated a case as positive if there was positive staining of more than 30% of the intraepidermal and or dermal infiltrate demonstrated positivity and found that the percent of positive cases increased from patch to plaque to tumor stage MF and was the greatest in SS. Interestingly, however, the actual percent of positive staining cells was similar in all groups being in the realm of 60–70% of the entire infiltrate. They did not specifically address if cases of patch stage MF showing a positive result for PD1 had a more aggressive clinical course 26. In another study, authors found a decrease in PD1 expression with tumor progression with noticeable absence of PD1 expression in large neoplastic cells in lesions of tumor stage MF 27. The results are somewhat similar to the lack of PD-1 expression in patients with advanced SS with high tumor burden, potentially reflecting the resistance of neoplastic cells to T-cell receptor activation, a critical event that leads to PD-1 expression. In our own unpublished experience, we found minimal PD1 expression in prelymphomatous T-cell dyscrasias which typically exhibit an indolent clinical course with its greatest expression in the setting of SS, an aggressive form of T-cell lymphoma 27.
Due to the immunosuppressive effects of PD-1, it would seem that tumor progression would be associated with enhanced PD-1 expression. The deleterious effects of the immunosuppression are not only with regard to susceptibility to infections but related to its inhibitory effects on counter regulatory lymphocyte populations designed to attenuate tumor growth and progression. The clinical progression of MF from skin-limited disease to more systemic involvement often correlates with the transformation of small neoplastic lymphocyte to those exhibiting a large cell morphology, a phenomenon well exemplified by this case 28. The definition of large cell transformation in the setting of MF is one based on a histomorphologic assessment, revealing a large cell infiltrate that exceeds 25% of the entire cell population and forms microscopic nodules. Large cell transformation can be accompanied by the acquisition of cytotoxic markers and changes in chemokine profile 29. CD30 positivity may or may not be a feature of the transformed cell populace. A recent study showed that CXCR3 while present in low-grade MF is absent in large cell transformation. The variable expression of CXRC3 and CLA in skin-limited versus systemic disease may provide a useful clue about clinical course. In the present case, the lack of CLA staining in the initial biopsy of plaque stage MF may have foretold the patient's eventual rapid and aggressive disease. Large cell transformation is an important finding that can signify disease progression. There are independent prognostic variables that may predict even a worse prognosis namely if the large cell transformation is in the context of concomitant folliculotropism, generalized disease rapid onset of large cell transformation from the time of initial diagnosis and finally CD30 negativity 30. Another study found that patients with de novo advanced stage disease demonstrated a better outcome than those patients who had a prior diagnosis of early stage MF. Transformation rate in patients with early-stage disease was less than 2% emphasizing its rarity in this particular clinical setting as opposed to the higher incidence in advanced tumor stage MF 31.
The treatment strategy for this patient is particularly interesting. Despite the aggressive nature of his disease, he achieved a complete response after 4 cycles of a novel combination of brentuximab vedotine and DICE chemotherapy. Since he had no HLA matched related or unrelated donors, he was enrolled on our alternative graft source stem cell transplantation protocol. The transplant conditioning was fludarabine and melphalan and the graft versus host disease prophylaxis was rabbit antithymocyte globulin and tacrolimus. We utilized an unrelated umbilical cord blood and CD3 selected stem cells from his son.
In conclusion, MF while considered under the general rubric of indolent T-cell lymphoproliferative disease can exhibit an aggressive clinical course manifesting tumor stage progression and dissemination to extracutaneous sites. The difference in the clinical course reflects a deviation in the normal pathobiology of MF. Likely oncogenic hits may develop under rare circumstances early in the clinical course leading to an accelerated progression reflective of a pathobiology that is typically seen only in advanced MF. In any case of seemingly early stage MF, careful evaluation for certainly clues such as large cells exhibiting angiocentricity with minimal epidermotropism, positivity for PD1, especially when in excess of 70% and a loss of CLA expression profile, should be considered as part of the diagnostic evaluation of the case. Perhaps such cases should be more aggressively managed including using targeted anti-PD-1 therapy.
Conflict of Interest
None declared.




