Biological Efficiency of Kunzea ericoides Based On Bioactive Compounds and Impact of Extraction
Abstract
New Zealand is known for its diverse population of flora and fauna, of which 80 % are endemic. Māori, the indigenous people of New Zealand, have profound and holistic knowledge of plants and utilize them in medicinal, spiritual, and ecological practices. Among these, kānuka has traditionally been used for medicinal purposes. Prior in vitro studies on kānuka extracts have demonstrated promising antioxidant, antimicrobial, anti-inflammatory, and antiproliferative properties. These studies further recommend the translation of these findings into new medicines and commercial products. However, a significant knowledge gap regarding their therapeutic potential hinders their application in various industries. A deeper understanding of the biochemical composition of the extract and the mode of interaction to exert its bioactivity in the host is vital for achieving this. Hence, this review evaluates the bioactivities of kānuka in association with its bioactive compounds (polyphenolics and terpenoids) reported in the current literature. Knowing the critical role of extraction methodologies in determining bioactive composition, we highlighted their efficiency in the bioactivities of kānuka.
1 Introduction
Kunzea ericoides (A. Rich.) Joy Thompson, commonly known as kānuka, is an endemic New Zealand plant that belongs to the Myrtaceae family (Fig. 1). It is a fast-growing tree or shrub that grows up to 6–20 m in height and is found in a variety of habitats from coastal to low alpine regions. It readily colonizes cleared forest and pasture areas and is often misidentified as mānuka (Leptospermum scoparium). Compared to mānuka, kānuka leaves are softer, narrower (0.5–1.8 mm), and longer (4–25 mm) [1]. Despite these differences, both trees have been described as having similar therapeutic properties [2].

Māori, the indigenous people of New Zealand, have extensive knowledge of their native plants and are skilled in utilizing them for therapeutic healing. They regard kānuka as a taonga (a treasure) and use it to treat diseases and alleviate pain. Kānuka leaves are most commonly used to prepare medicinal beverages. The infusion was consumed for blood disorders, headache, flu, cough, sinus infection, hay fever, bronchitis, asthma, skin infection, and kidney disorders. In addition, young shoots were chewed as a remedy for the dysentery. The leaves are dipped into the oil to produce fragrant oil [3, 4].
Several in vitro biological studies inspired by mātauranga Māori (traditional knowledge and cultural practice of Māori [5]) have explored the potential of kānuka extract (solvent and distillation) for its anti-inflammatory [6], pharmacological [7], and antiviral properties [8]. However, the current use of kānuka is limited to essential oil (EO) production. Although several studies have proven the biological competence of kānuka EO (KEO), its elevated viscosity and aroma render it unsuitable for wider industrial applications.
The development of a plant-based product or the derivation of its active compound for specific applications is complicated. A deeper understanding of the biochemical profile, interactive ability with the host, and suitability for sustainable recovery is required. To achieve this, researchers have extensively investigated kānuka bioactive compounds (BCs) using various techniques, including organic solvents (ethyl acetate, methanol, and dichloromethane), distillation, and green extraction methodologies (subcritical water and ultrasound-assisted extraction [UAE]). These studies have identified diverse compounds, including terpenoids, flavonoids, and lipids (fatty acids and triglycerides), with promising biological properties, including antioxidant, antimicrobial, and antiproliferative [9-12]. These findings provide compelling evidence for the contribution of kānuka metabolites to drug development.
Despite growing interest in utilizing the benefits of kānuka in various sectors, such as industry and healthcare, there is still a lack of comprehensive research, limiting its widespread application. For example, the efficiency of kānuka extract has not been thoroughly compared to that of the synthetic analogs of its major BCs. This comparison is crucial to determine whether the natural extract (combination of various BCs) exhibits a synergistic effect that offers potential advantages in terms of biological properties or an antagonistic effect, which requires the isolation of biologically important compounds. In addition, the mechanisms by which these BCs exert their biological effects remain unclear. Addressing these gaps is essential for revealing the potential of kānuka in industrial applications.
Hence, this review primarily evaluates the biological properties of kānuka extract based on its functional group, notably flavonoids and phenolic acids, as reported in current in vitro studies. Then, the biological efficiency of the kānuka extract was compared to that of the synthetic analog to determine (i) the interaction (synergistic or antagonistic) of BCs in a biological system, (ii) the mode of pathogenicity, and (iii) the impact of extraction methodologies in determining the bioactive composition and biological properties of the extract. This review may encourage further investigation into the extraction and characterization of BCs and explore the potential implications of kānuka extracts in health and disease.
2 Kānuka Bioactive Compounds
BCs are secondary metabolites of plants that are secreted in response to biotic and abiotic environmental stresses. BCs are categorized based on their biosynthetic origins and include terpenoids, phenylpropanoids, and alkaloids [13]. Each group has distinct biological and physicochemical properties that facilitate extraction and identification. Polyphenols and terpenoids are most abundant in plants and have been increasingly preferred for drug discovery over synthetic metabolites because of their ability to interact with a diverse array of disease-causing proteins, prevent biofilm formation, and disrupt cytoplasmic membranes and nucleic acids [14, 15].
Although BCs are recognized for their numerous beneficial effects in humans, their cytotoxicity and nonselective interaction with the target can result in adverse effects. The literature on plant extracts primarily focuses on screening for biological activity, cytotoxicity, and quantification of BCs. This is crucial for promoting valuable properties and for identifying new metabolites. However, not all compounds retain their potential functionality after successful separation. Recent investigations on the physicochemical properties of BCs and their structural relationship with the target have revealed that some compounds exhibit multiple behaviors that interfere with many biological assays, resulting in a false-positive readout. These compounds are known to be frequent hitters or promiscuous compounds.
Several in silico filters have been used to filter promiscuous compounds, including the pan-assay interfering compounds (PAINS). PAINS contains molecules defined by a common substructural motif, which confers the ability to interfere with assays via various mechanisms, including thiol reactivity, metal chelation, redox activity, and micelle formation [16]. Many prominent BCs were found to exhibit promiscuous behavior when using PAINS. For instance, Baell [17] demonstrated the promiscuous behavior of BCs containing catechols, including epigallocatechin gallate and gossypol, which cause membrane disruption and cytotoxicity. Hence, qualitative determination of the extract, followed by in silico filtration, is necessary to select metabolites without nonspecific reactivity for further optimization.
Like other medicinal plants, kānuka BCs have shown in vitro bioactivities, such as myorelaxation, anti-inflammatory, antiproliferative, antibacterial, and antioxidant activities, associated with different biochemical profiles, including phenolic acid, flavonoids, terpenoids, and alkaloids [8, 10, 11, 18]. In addition, they have been shown to maintain soil hygiene and prevent wood decay. For example, root exudates of kānuka reduced Escherichia coli counts in soil containing organic waste by 90 % within five days of incubation [19]. Similarly, KEO (1 % w/v) inhibits the growth of the brown root fungus Coniophora puteana [20]. The relationship between the identified BCs of kānuka, based on their functional groups (terpenoids and polyphenolic compounds [PCs]), and their biological properties are discussed further in this section.
2.1 Bioactivities of Kānuka Terpenoid Compounds
The Myrtaceae family is characterized by oil glands in all parts of the plant that secrete aromatic EO. The predominant constituent of EO is terpenoids (isoprenoids). Terpenes are a diverse class of natural products consisting of hydrocarbons and oxygenated derivatives with functional groups such as hydroxyl, carboxyl, ketone, or aldehyde groups. These molecules are synthesized via the mevalonate or methyl-d-erythritol 4-phosphate pathway. Precursors, including isopentenyl pyrophosphate and dimethylallyl pyrophosphate, produce intermediate molecules (geranyl pyrophosphate, farnesyl pyrophosphate, and geranylgeranyl pyrophosphate) that are subsequently converted into a vast array of terpenoids by terpene synthase [21].
Studies on KEO obtained from different geographical locations within New Zealand have reported 42–73 metabolites, predominantly monoterpenes [22]. Apart from α-pinene (34–74 %) [18, 23] as the primary compound, it contains a minor proportion of globulol (18.4 %), betulinic acid (8.1 %) [10], 1,8-cineole (4–6 %), viridiflorol (1.5 %), spathulenol (1.35 %), and p-cymene (2.9 %) (Fig. 2) [22, 24]. The impact of environmental factors in determining the concentration and composition of kānuka terpenoid compounds has been reported by Maddocks [22] and Van Vuuren et al. [18]. The significant observations were as follows: (i) Variations in the concentration of α-pinene. Maddocks [22] reported that KEO obtained from five different geographical regions (within New Zealand) showed a 13.42 % variation in α-pinene constituents (with the highest concentration, 74 %, found in the Great Barrier Island, New Zealand). In contrast, Van Vuuren et al. [18] found that KEO from Limpopo Province, South Africa had α-pinene concentrations of 30–46 %. (ii) Seasonal variations caused inconsistencies in the occurrence of minor compounds. For instance, the concentration of p-cymene was significantly reduced (8.9 %) in the samples harvested at the end of the year (November and December) compared to the middle of the year (July and September—16.6–18 %) [18]. (iii) Genetic drift or selective pressure in different regions may lead to changes in the secretion of minor compounds. For example, compounds, such as p-cymene (5.82 %), α-selinene (4.13 %), cadina-3,5-diene (2.74 %), and methyl geranate (2.12 %), identified in the historical reference kānuka sample (2018, Otago, South Island, New Zealand), were not detected in other samples obtained during 2019 and 2020. Additionally, temperature, soil conditions, water quality, and pollution may influence terpenoid secretion in plants [25, 26]. Based on compositional variations, KEO exhibited different biological properties (Tab. 1).
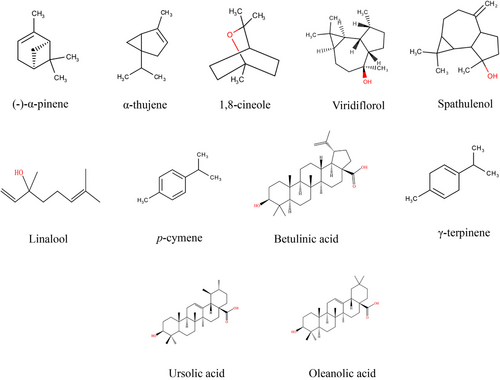
| Bioactivity | Organisms/cell lines | Method | Test concentration (v/v) | Observation | References |
|---|---|---|---|---|---|
| Antimicrobial | Staphylococcus aureus, Staphylococcus epidermidis, Mycobacterium smegmatis, Enterococcus faecalis, Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus pneumoniae, Propionibacterium acnes, Brevibacterium brevis, Brevibacterium agri, Brevibacterium laterosporus, Moraxella catarrhalis, Pseudomonas aeruginosa, Klebsiella pneumoniae, Cryptococcus neoformans and Candida albicans | BMD | 1–32 mg mL−1 | Brevibacterium species and C. neoformans were sensitive at MIC 1 mg mL−1. MIC > 2 mg mL−1 was required to inhibit other pathogens | [18] |
| S. aureus, Streptococcus mutans, Streptococcus sobrinus, Escherichia coli | BMD | 10 % (v/v) | The inhibitory effect was observed on all pathogens within 5 s to 15 min of exposure to the oil | [6] | |
| S. aureus | Death kinetic | 0.25–2.0 % (v/v) | Bactericidal activity was not observed | [23] | |
| S. aureus | Vapour disc | 5–0.0391 µL mL−1 | KEO vapor exhibited bacteriostatic and bactericidal effects at MIC 0.3125 mg mL−1 and MLC > 0.625 mg mL−1, respectively. | [27] | |
| 25 different bacteria and 20 different strains of Listeria monocytogenes | Well-diffusion | 10 µL | L. monocytogenes was sensitive to KEO. The ZI was observed for S. aureus, Streptococcus faecalis, Micrococcus luteus, and Bacillus subtilis as 10.8, 5.9, 5.0, and 6.3 cm in diameter, respectively | [28] | |
| S. aureus, E. coli | BMD | 10–1.25 mg mL−1 | The terpene extract inhibited the growth of S. aureus at MIC 2.5 mg mL−1 was not against E. coli | [10] | |
| S. aureus, Bacillus cereus, E. coli | Disc diffusion | 10 µL | The oil produced ZI of 9.7, 10.3, and 6 mm in diameter for S. aureus, B. cereus, and E. coli, respectively | [24] | |
| Trichosporon mucoides, Malassezia furfur, C. albicans, Candida tropicalis | BMD | 0.01–12.5 % | The oil inhibited the growth of T. mucoides and M. furfur at MIC 0.78 % and Candida species at MIC 3.18 % | [6] | |
| Aspergillus niger, Aspergillus ochraceus, Fusarium culmorum | Broth dilution | 1 µL mL−1 | Pathogens were resistant to KEO | [28] | |
| Antioxidant | Beta-carotene, linoleic acids, and bacto-agar | Well-diffusion | 100 µL | The bleaching effect was 17.7 mm wide with the KEO from North Island | [28] |
| Antitumor | Mouse lymphoblastic parental P388 leukemia | TBE | 1, 10, 100 µg mL−1 | Dose-dependent cytostatic and cytotoxic activity was observed. At 100 µg mL−1, terpene extract exhibited a 40 % reduction in tumor growth | [10] |
| Cytotoxic | Rat hepatocytes | TBE | 1, 10, 100 µg mL−1 | More than 50 % of cell death was observed | [10] |
| Vero and RC-37 | Neutral red | 0.0001–1 % | Noncytotoxic concentration was 0.0001 %, whereas 0.0043 % has reduced >50 % cell viability | [29] | |
| Anti-inflammatory | THP-1 | XTT-cell proliferation | 0–10 % | The oil has reduced TNF-α (<10 ng mL−1) release and has not altered the effect of interleukin-4 | [6] |
| Porcine neutrophils | leukotriene liberation | 10 µg mL−1 | The terpene extract and KEO have inhibited the leukotriene production by 72 % ± 8 % and 51 % ± 6 %, respectively | [10] | |
| Myorelaxation | Guinea-pig ileum | 0.1 Hz, 0.5 ms, 70 V | >200 µL | At 6 × 10−6 g L−1 concentration, the spasmogenic and spasmolytic activity was observed | [30] |
| Male albino rat diaphragm | 0.21 Hz, 0.5 ms, 40 V | 2 × 10−3 v/v | The spasmogenic activity slowly increased, followed by a decrease in tension | [7] | |
| Chick biventer muscle | 0.21 Hz, 0.5 ms, 40 V | 1 × 10−3 v/v | Decreased tension or semi-contraction was observed | ||
| Rat uterus | 0.1 Hz, 0.5 ms, 70 V | 1 × 10−6 v/v | 100 % spasmogenic activity was observed |
2.1.1 Antimicrobial Property
Kānuka EOs exhibit enhanced antimicrobial activity because of their lipophilic nature, which has a higher affinity toward the cell membrane and affects the structure by interacting with membrane components, including proteins, fatty acids, and phospholipids [15, 24, 31, 32].
Staphylococcus aureus and E. coli are the most preferred pathogens for antibacterial screening of plant extracts due to their clinical importance, well-characterized biology, high virulence, and resistance to multiple antibiotics [33]. Monoterpene analogs, such as α-pinene (MBC 3.432 mg mL−1) and limonene (0.421 mg mL−1), have demonstrated effectiveness against these reference organisms [34]. Hence, monoterpene-enriched EOs are expected to exhibit antibacterial activities. However, several studies have observed either minimal or dose-dependent inhibition of KEO. For instance, Wyatt et al. [10] and Van Vuuren et al. [18] found that, at lower concentrations, KEO (MIC > 8–10 mg mL−1) and solvent extract (MIC 2.5 mg mL−1) exhibited minimum inhibition against S. aureus and were ineffective against E. coli (MIC >10 mg mL−1). Conversely, Chen et al. [6] reported complete (100 %) inhibition of pathogens (S. aureus, E. coli, S. mutans, and S. sobrinus) at high concentrations (100 mg mL−1). However, the lack of information provided on the bioactive composition in this study [6] makes it challenging to compare the antibacterial properties of KEO with those of other studies [10, 18].
Although KEO has not produced satisfactory inhibition of reference organisms, it has been shown to be effective against (in vitro) gram-positive pathogens, including L. monocytogenes (KEO 10 µL) [28] and Brevibacterium (MIC 1 mg mL−1) [18]. It demonstrates the selective antibacterial activity of KEO. According to Seltmann and Holst [35], the composition and structure of gram-positive cell walls differ, whereas the gram-negative cells follow a more general structure. The cell wall is a multifaceted structural barrier for survival and environmental interactions, providing a primary target for drug development. The cell envelope of gram-positive bacteria consists of a thick peptidoglycan layer. In contrast, gram-negative bacteria have a thin peptidoglycan surrounded by an outer membrane containing a bilayer with an outer leaflet of lipopolysaccharide, which provides a strong barrier to penetration. Due to the lack of this outer membrane, gram-positive bacteria are more susceptible to antibiotics. Additionally, molecular analysis studies have identified that terpenes, including α-pinene, carvacrol, limonene, and eugenol, actively participate in inhibiting the efflux pump (a protein complex located in the cell membrane, which facilitates the transfer of a toxic substance from the bacterial cell to survive against antibiotics) in multidrug-resistant gram-positive bacteria compared to gram-negative bacteria [32].
One commercial application of KEO is in the treatment of dermatophytes that cause skin, scalp, and nail infections. It has been added as an antimicrobial ingredient in the formulation of soap, creams, and toothpaste and applied externally in aromatherapy and internally by herbalists [7]. Although α-pinene (analog) has been observed to produce pathogenicity by inhibiting pseudo-hyphae formation [24, 36], the antifungal efficacy of KEO has not been consistently demonstrated. For example, KEO inhibited the growth of Trichosporon mucoides and Malassezia furfur (in vitro, MIC 7.8 × 103 µg mL−1) at low concentrations compared to Candida albicans and Candida tropicalis (MIC 3.18 × 104 µg mL−1) [6]. This outcome is significantly higher than that of the effect of α-pinene analog, which kills C. albicans (Minimum fungicidal concentration (MFC) 64 µg mL−1) and Candida parapsilosis (MFC 128 µg mL−1) [36]. These results support the hypothesis that minor constituents influence the biological activity of the extract. The impact of minor compounds and their concentrations on in vitro antifungal activity has been well documented by Maddocks et al. and Maddocks [22, 37]. According to their study, α-pinene alone is insufficient to inhibit mycelial growth. In addition, the KEO from Great Barrier Island, containing β-selinene (1.62 %), effectively inhibited the growth of Trichophyton rubrum (IC50 0.67 %) and Microsporum canis (IC50 2.7 %), compared to other KEO.
In conclusion, KEO exhibits antimicrobial properties with lower efficacy than commercial antibiotics, including ampicillin and amphotericin B. Furthermore, its antiseptic property may not be ascribed to killing or inhibiting a broad spectrum of pathogens but selective pathogens.
2.1.2 Myorelaxation Activity
KEOs are preferred for aromatherapy because of their unique characteristics. It is often used in combination with massage therapy, where inhalation of oil vapor generates a beneficial effect via the hormonal and limbic systems of the brain [38]. Studies have shown that an α-pinene analog (10 µL L−1) exhibits (in vitro) anxiolytic-like activity with reduced accumulation in the mouse brain and liver after 5 days of continuous inhalation [39]. According to Lis-Balchin and Hart [30] and Lis-Balchin et al. [7], monoterpenes in KEO, including α-pinene, α-terpinene, and β-pinene, may influence muscle tension through the myogenic pathway. In contrast, sesquiterpenes such as α-terpineol and terpinene-4-ol may participate in muscle relaxation.
The myorelaxation activity of KEO has been demonstrated in different types of tissues, including smooth and skeletal muscles. Studies have shown dose-dependent activity in isolated (in vitro) guinea pig ileum. At low concentrations (IC50 6 × 10−6 g L−1), KEO exhibited minimum inhibition of contraction (spasmogenic), whereas at high concentrations (IC50 5 × 10−5 g L−1), the spasmolytic activity was rapid and extended for up to 12 min [30]. Consequently, these findings indicate the importance of determining the dosage and oil composition for aromatherapy, which is essential for obtaining the therapeutic potential of KEO.
2.1.3 Anti-Inflammatory Property
Inflammation is a protective response to various pathogens and tissue injuries. It is produced and controlled by the inducible transcription factor, nuclear factor kappa B (NF-kB). NF-kB regulates genes responsible for inflammatory agents (cytokines and chemokines) and promotes the activation and differentiation of naïve T cells (Th1 and Th17) to mediate the inflammatory response. Upon encountering pathogens or tissue damage, a series of downstream cascade mechanisms activate NF-kB. Based on its activation mode, NF-kB can trigger the production of proinflammatory and anti-inflammatory cytokines. However, aberrant NF-kB activation or deregulation of inflammation may lead to degenerative diseases, including rheumatoid arthritis and osteoporosis. Hence, a biomolecule that inhibits NF-kB holds promise as a potential treatment for inflammatory diseases [40]. Interestingly, terpenes found in KEO may offer potential inhibitory effect on NF-kB activation.
Monoterpenoids exert anti-inflammatory effects by inhibiting the signaling pathway [41], reducing proinflammatory mediators, and modulating cytokine expression [41]. Several in vitro studies have investigated the anti-inflammatory properties of α-pinene and KEO, highlighting their impact on NF-kB inhibition and reduction in cytokine release (tumor necrosis factor-α [TNF-α] and interleukin 4 [IL-4]) and proinflammatory mediators (leukotriene B4 [LB4]). For instance, α-pinene analogs suppress (in vitro) inflammation by inhibiting the translocation of NF-kB into the nucleus and increasing the expression of a protein (IkBα) in THP-1 (human acute leukemia monocytes) cells (Fig. 3) [42]. Additionally, Kim et al. [43] reported that α-pinene (20 µM) regulates anti-inflammatory activity through various pathways, including the production of IL-6, TNF-α, and nitric oxide, inhibition of nitric oxide synthase and cyclooxygenase-2 enzymes, and prevention of the binding of NF-kB to deoxyribonucleic acid (DNA), in LPS (200 ng mL−1)-induced macrophage cells (mouse peritoneal).
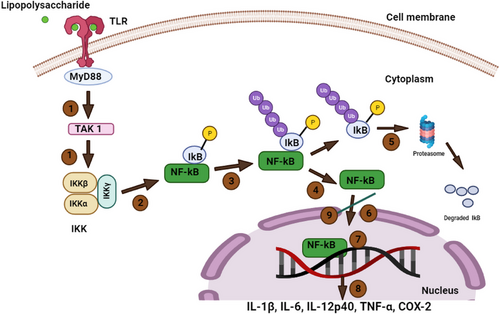
Likewise, Wyatt et al. [10] observed that KEO (10 µg mL−1) reduced LB4 secretion of (in vitro) porcine neutrophils (pig blood) induced by arachidonic acid (1 mM). In addition, this study demonstrated different degrees of reduction in LB4, based on the major component. KEO rich in globulol (18.4 %) inhibited LB4 secretion by 51 %, whereas the terpene extract containing betulinic acid (8.1 %) inhibited 72 % compared to the control group (nordihydroguaiaretic acid 89 %). These findings suggest that the KEO components strongly influence the regulation of anti-inflammatory mediators. Similarly, Chen et al. [6] demonstrated the selectivity of KEO (1 %) in inhibiting the expression of TNF-α (<10 ng mL−1), an inflammatory mediator, without altering IL-4 expression (associated with allergic response) in THP-1 cells treated with lipopolysaccharide (0.2 µg mL−1). This selectivity offers safety for dermal applications, which is an added advantage when considering alternatives to synthetic drug metabolites.
2.1.4 Antiproliferative and Cytotoxicity
Cell division is essential for tissue growth and can be grouped into two phases: interphase and mitosis (Fig. 4). Proteins that regulate cell division, including cyclin-dependent kinase (CDK) and cyclin, are regulated by the expression of two tumor suppressor genes, p53 and pRB. p53 prevents genetic mutations that cause cancer, whereas pRB regulates cell cycle. Mutations in these gene sequences may lead to uncontrolled proliferation, resulting in tumor development. Therefore, these mutated gene sequences are primary targets for developing antiproliferative drugs [44]. Monoterpenoids, including α-pinene, limonene, and linalool, exert multifaceted interactions with cellular pathways to inhibit the proliferation of cancer cells [45-47].
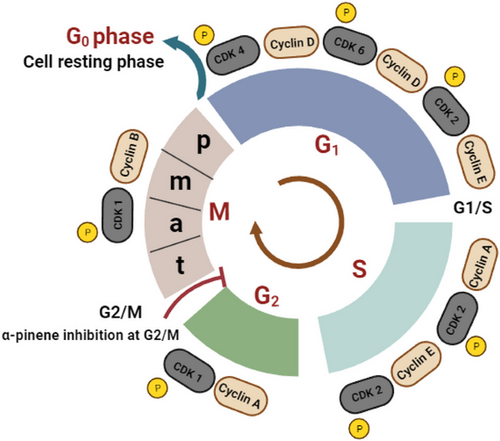
Many studies have investigated the antiproliferative activity of α-pinene and KEO. Wyatt et al. [10] observed that KEO (10 µg mL−1) partially (40 %) inhibited the growth of leukemia cells (mouse lymphoblastic parental P388 tumor cells) without initiating apoptosis. The mechanism underlying this inhibition has not yet been elucidated. However, these findings were consistent with those reported by Chen et al. [45]. They observed that α-pinene (IC50 8.4 mM, pine leaves) exhibited antiproliferative activity in human hepatoma carcinoma (BEL 7402) cells by arresting the cell cycle at the G2/M phase and decreasing CDK1/cyclin B1 expression without promoting cell death (Fig. 4). Similar to α-pinene, d-limonene (4 mM) mediates its antiproliferative activity by inducing cell cycle arrest (G1/M phase), which is mediated by the downregulation of the CDK1/cyclin B1 complex in a human breast cancer cell line (in vitro MCF7). This disruption leads to mitochondrial dysfunction that triggers apoptosis. Molecular interaction studies have revealed that d-limonene directly interacts with the protein (CDK1/cyclin B1) at the molecular level, influencing structural and functional integrity [46]. In contrast, Pushpalatha et al. [47] observed that linalool (IC50 43 µM) exerted dose- and time-dependent antiproliferative effects by generating reactive oxygen species in human oral carcinoma KB cells. This leads to a series of events, including a decrease in mitochondrial membrane potential and induction of apoptosis characterized by chromatin condensation and nuclear fragmentation. These studies indicate that metabolites of kānuka offer a potential opportunity to treat various cancers.
The determination of cytotoxicity is essential for the development of naturally derived drug molecules. Studies have investigated the cytotoxicity of KEO using various cell lines, including rat hepatocytes, THP-1, Vero, and RC-37 (a kidney epithelial cell line from the African green monkey). These studies indicated that KEO is highly toxic at low concentrations. For instance, KEO and terpene extract (10 µg mL−1) reduced cell viability by 50 % in rat hepatocytes [10], and Vero and RC 37 (KEO 0.0043 %) [10, 29]. This observation aligns with the outcome of Schnitzer et al. [29], where the noncytotoxic concentration of α-pinene was reported to be 0.0001 % in the RC-37 cell line. Interestingly, Chen et al. [6] observed no impact on cell viability (THP-1) at 10 % concentration of KEO. This implies that the toxic potential of kānuka is dose-dependent, and the concentration varies depending on the type of cell line.
2.1.5 Insecticidal Property
Terpenoids are well-known for their insecticidal properties. According to Boulogne et al. [48], it contributes 37 % of its insecticidal properties compared to other phytochemicals. Although the exact interaction between metabolites and insects remains unclear, some studies indicate that they produce neurotoxicity via a cholinergic and octopaminergic pathway, influencing the behavioral pattern and leading to death [49].
The insecticidal properties of KEO have been investigated in various insect species. Kassimi et al. [50] found that KEO (0.2 %) induced 50 % mortality (LC50) in green alfalfa aphids. This effect was comparable to the synthetic insecticide, Malyphos, which exhibited 50 % mortality at lower concentrations (0.1 %). KEO showed greater potency at 1 % concentration, reaching 90 % mortality within 13.5 h (TL50). In addition, the hexane kānuka extract and KEO-containing ficifolidione have shown effectiveness against Musca domestica (44 µg insect−1), Culex pipiens (1.2 µg insect−1), Aphis fabae (LD50 12 µg insect−1), and Thrips tabaci (LD50 5.9 µg insect−1) [51, 52]. These studies indicate that the metabolites of kānuka can be environmentally safe alternative insecticides while maintaining efficacy for extended periods.
2.1.6 Interactive Potential of Kānuka Essential Oil with Other Essential Oils
The phytochemical composition of plant extracts is unique, and different components may contribute to various biological effects. However, when the two extracts were mixed, they exhibited additive, synergistic, and antagonistic activities. Additive interaction occurs when the combined activity equals the sum of the individual activities. When the activity is less than or greater than the additive, it is known to be antagonistic or synergistic, respectively [53]. KEO exhibited synergistic and antagonistic effects when combined with other EOs, including Leptospermum petersonii oil and mānuka EO (MEO) (Tab. 2). For instance, when two active KEO and MEO were mixed (1:1), antagonistic and synergistic effects were observed, with reduced antioxidant and increased spasmogenic activity, respectively [28].
| Essential oil | Organisms | Method | Test ratio | Observation | References |
|---|---|---|---|---|---|
| LEO, MEO, and KEO | S. aureus, P. aeruginosa, C. albicans | BMD checkerboard | 9:1 to 1:9 |
LEO: KEO, additive effect was observed at all the tested ration on three pathogens MEO: KEO, additive effect was observed against P. aeruginosa and C. albicans at all ratios |
[18] |
| KEO and MEO | A. niger, A. ochraceus, F. culmorum | Broth dilution | 1:1 | An antagonistic effect was observed, where the inhibition potential of MEO was reduced from 64 %, 49 %, and 25 % to 30 %, 13 %, and not effective on pathogens, A. niger, A. ochraceus, and F. culmorum, respectively | [28] |
| KEO and MEO | Radiation-induced mucositis | Randomized placebo-controlled trial | 1:1 | The development of RIM was observed two times slower in the active group than in the placebo untreated group | [54] |
Preclinical studies have shown that KEO and MEO share therapeutic properties that reduce the development of radiation-induced mucositis (RIM). RIM is described as inflammation of the oral and oropharyngeal mucosa caused by radiation when treating head and neck cancer. According to Maddocks-Jennings et al. [54], the combined effect of KEO and MEO (1:1 drops diluted with 10–15 mL of warm tap water) as a gargling solution significantly delayed the onset of mucositis for 15.6 days compared to the control group that received usual oral care (10.8 days) and the placebo group treated with distilled water (7.25 days) (Tab. 2). Additionally, the outcomes of reduced pain and associated health effects, including weight loss and oral symptoms, correlate with the in vitro bioactivity of KEO, as observed in other studies [6, 8, 10].
2.2 Bioactivities of Kānuka Phenolic Compounds
PCs constitute the second-largest group of phytochemicals. They are synthesized via shikimic acid and malonic pathways, providing structural support and protection against ultraviolet solar radiation. They are grouped as flavonoids and phenolic acids. Flavonoids contain two phenyl rings (A and B) joined via a heterocyclic pyran ring, whereas phenolic acids contain a single phenyl group substituted by one carboxylic group or one or more hydroxyl groups. PCs scavenge free radicals to neutralize their harmful effects, prevent non-transmissible chronic diseases (cardiovascular disease and cancer), and regulate cellular processes (enzyme inhibition and phosphorylation) [55].
The solubility of PCs varies depending on the number of hydroxyl or other functional groups. Polar solvents, such as methanol and ethanol, were used to estimate the total phenolic content of the plant matrices. In kānuka, few studies have explored PCs using organic solvents and subcritical water extraction (SWE) (Fig. 5). Compared to organic solvents, SWE provides more information about PCs [11]. However, water solubility at different temperatures may raise concerns regarding whether the extract contains only PCs. The highest temperature used for SWE in kānuka so far is 210 °C [11], where the polarity of water remains equal to that of methanol (ε = 32.6) [56]. It indicates that the extract might contain more PCs than terpenoids. The bioactivity of kānuka, based on the presence of PCs, is presented in Tab. 3.
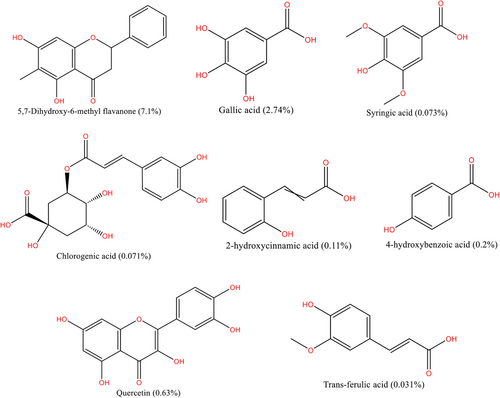
| Bioactivity | Organisms/cell lines used | Method used | Test concentration | Observation | References |
|---|---|---|---|---|---|
| Antiviral | Herpes simplex Type 1, Polio Type 1 | Disc diffusion | N/A | The extracts inhibited the growth of pathogens at 5 and 40 µg disk−1, respectively | [8] |
| Antibacterial |
S. aureus E. coli |
BMD | N/A | The methanol extract has inhibited the growth of S. aureus (MIC ≥ 10 mg mL−1) but is ineffective on E. coli | [10] |
| BMD | 50–0.78 % | The most effective fraction was observed at 210 °C, MIC 4.4 mg mL−1 on both organisms | [57] | ||
| 50–80 % | The bacteriostatic effect was observed at 60 % ethanol (MIC 7.5 mg mL−1) extract on S. aureus and ineffective on E. coli | ||||
| Antitumor | Mouse lymphoblastic parental P388 leukemia | TBE | 1, 10, 100 µg mL−1 | The extract has not exhibited a cytostatic effect nor produced a toxic effect on cells | [10] |
| Murine fibroblast (L cells), human rhabdomyosarcoma (RD-cells), human cervix carcinoma (Hep-2) | MTT | 6.25–10 mg mL−1 | The extract caused 80 % death of L cells, Hep-2 cells, and 55–70 % of RD cells. The most effective fraction was observed at 210 °C, IC50 216–389 µg mL−1 | [57] | |
| Cytotoxicity | Rat hepatocytes | TBE | 1, 10, 100 µg mL−1 | Cytotoxicity was not observed at all tested concentrations of flavonoid glycoside fraction | [10] |
| Antioxidant | Degradation of deoxyribose | 100 µg mL−1 | The extract has reduced 20 % of sugar in deoxyribose | [10] | |
| DPPH | 10 µL | The methanol extract has reduced the DPPH at IC50 9.579 µg mL−1 compared to ethyl acetate extract, IC50 29.59 µg mL−1 | [12] | ||
| DPPH and FRAP | 100 µL | The maximum activity was observed at 170 °C as 109.78 and 246.37 mg TE g−1 dw for DPPH and FRAP assay, respectively | [11] | ||
| Enzyme-inhibitory | Acetylcholinesterase and butyrylcholinesterase | Ellman's colorimetry | 1000 µg mL−1 | The methanol extract inhibited 96 % of β-secretase (IC50 29.05 µg mL−1) and 75 % of AChE and BChE enzymes (IC50 215.4, 361.6 µg mL−1) | [12] |
| Beta-secretase | Recombinant human BACE1 | 500 µg mL−1 |
- Abbreviations: BMD, broth microdilution; DPPH, 2,2-diphenyl-1-picrylhydrazyl assay; FRAP, ferric reducing antioxidant power; TBE, trypan blue exclusion.
2.2.1 Antioxidant Property
Studies on kānuka's antioxidant properties have shown a consistent positive correlation between the concentration of PCs and antioxidant activity. For example, the PCs of kānuka methanol extract containing flavonoid glycosides (19.56 %) inhibited (in vitro) 20 % oxidation of sugar in deoxyribose [10]. Similarly, kānuka SWE exhibited the highest antioxidant activity (DPPH: 223.8 mg ± 2.7 mg TE g−1 dw; ferric reducing antioxidant power (FRAP): 478.8 mg ± 2.1 mg TE g−1 dw) at 170 °C, where the highest total phenolic content (TPC) (172.8 mg ± 2 mg GAE g−1 dw) was obtained. Compounds, including gallic acid, catechin, and quercetin [11], which contain hydroxyl groups, may have influenced the antioxidant activity. Owing to their structural chemistry, polyphenols exhibit greater antioxidant activity than vitamins (E and C). They serve as free radical scavengers and stabilize and neutralize harmful effects by donating hydrogen and electrons. In addition, it actively participates in metal chelation and prevents free-radical formation. These studies demonstrate that kānuka can be a nutritional supplement that aids in health benefits and disease prevention associated with oxidative damage [58].
2.2.2 Antibacterial Property
The antibacterial properties of PCs are based on their physical and chemical characteristics, including lipophilicity and charge. These properties can disrupt the integrity of the cell membranes of pathogens, leading to cell death [59]. Studies have shown that kānuka extracts obtained via the solvent and SWE methods exhibit varying degrees of growth inhibition against pathogens, including S. aureus and E. coli. For example, a solvent extract containing 5,7-dihydroxy-6-methyl flavanone (12.1 %) was not significantly effective against either pathogen (MIC > 10 mg mL−1) [10]. In contrast, the SWE extract (210 °C) containing syringic acid exhibited higher bactericidal activity (MIC 4.4 mg mL−1) than the extract (170 °C) containing gallic acid (MIC 7.5 mg mL−1) [57]. This difference might be due to the presence of hydroxybenzoic acids and the increased acidic conditions at 210 °C. Studies have suggested that the functional groups and pH of extracts play a crucial role in determining the antibacterial properties of PCs. For instance, Sánchez-Maldonado et al. [60] observed (in vitro) 10-fold greater antibacterial activity against lactic acid bacteria with compounds containing methoxy functional groups and an acidic pH of the medium. The kānuka BCs exhibited antibacterial activity when applying suitable extraction parameters, including solvent type and temperature.
2.2.3 Antiviral Property
One of the promising biological properties of the Myrtaceae family is their activity against viral pathogens owing to phloroglucinol (PG) [8]. PG (trihydroxybenzene) and its derivatives are excellent antiviral agents against Enterovirus (EV71) and Human immunodeficiency virus [61]. The antiviral mechanism of PG derivatives is believed to involve interactions with viral proteins (polymerases and proteases). By interfering with these proteins, PG can disrupt viral replication and prevent the virus from infecting the host cells [62]. Bloor [8] investigated the in vitro antiviral activity of polar and nonpolar fractions of kānuka. The author found that both extracts exhibited cytopathic effects against Herpes simplex Type 1 and Polio Type 1 viruses. Structural analysis of the active metabolites revealed four different acyl-PG molecules that inhibited the viruses at 5 and 40 µg disc−1. This study supports the traditional medicinal use of kānuka leaves against viral infections and suggests that it is a promising source of antiviral biomolecules.
2.2.4 Therapeutic Potential for Alzheimer's Disease
Alzheimer's disease (AD) is a neurological disorder that is characterized by dementia. Oxidative stress is a major contributor to the production of the amyloid beta protein, which disrupts brain function. Hence, the development of drugs that inhibit the activity of enzymes, including acetylcholinesterase, butyrylcholinesterase, and β-secretase, to retard AD progression is important [9]. Several flavonoids have been demonstrated to interact with the active site of β-secretase. For example, quercetin (5.4 µM) inhibits β-secretase by forming one hydrogen bond with Asp32, the catalytic active site in the enzyme [63]. Similarly, Majid and Silva [12] found that kānuka inhibited more than 70 % of all the three enzymes. In particular, the methanol extract (IC50 14.25 µg mL−1) inhibited 90–95 % of β-secretase. Although the BCs of the extract were not analyzed, it is believed that the presence of quercetin in kānuka, as reported by Essein et al. [11], might have induced an enzyme inhibitory response. Understanding the composition of SWE and its mechanism of action may offer a potential therapeutic solution for AD.
2.2.5 Antiproliferative and Cytotoxic Properties
Phenolic compounds exert both antioxidant and pro-oxidant effects. Prooxidants contribute to antitumor effects by inducing oxidative stress through the redox activity of Cu(II). Li et al. [64] observed that PCs reduce Cu(II) to Cu(I) in the presence of oxygen, forming a free radical-PC complex that binds to DNA and induces strand cleavage. Hence, the antiproliferative potential of PCs is regarded as their ability to bind metal ions and the contribution of the hydroxyl functional group rather than the methoxy group.
Although few studies examine the antiproliferative activity of kānuka phenolic compounds, the mechanism underlying their inhibitory effect remains unexplored. The kānuka flavonoid glycoside (100 µg mL−1) compounds did not inhibit the proliferation of leukemia cells or induce toxicity in rat hepatocytes [10]. According to Kawaii et al. [65], flavonoid glycosides (40 µM) are not regarded for their antiproliferative properties and do not exert cytotoxicity in vitro against human non-transformed cell lines (umbilical vein endothelial [HUVE] and foreskin keratinocytes [HFK]). Similarly, the kānuka subcritical water extract (6.5–10 mg mL−1) has demonstrated time and dose-dependent activity in various cancer cell lines, including murine fibroblasts (L cells: IC50 216.8 ± 3.4 µg mL−1, 210 °C), human cervix carcinoma (Hep-2: IC50 323.6 ± 20.8 µg mL−1 190 °C) and rhabdomyosarcoma (RD-cells: IC50 309.7 ± 16.1 µg mL−1). The extracts prepared at 210 °C have inhibited 80 % growth of the cell lines, including L cells and Hep-2 cells. This effect may be attributed to the compounds with hydroxyl groups, including trans-ferulic acid and chlorogenic acid [11, 57]. PCs’ cytotoxic and selective antiproliferative effects are associated with their chemical structure. Selassie et al. [66] show that PCs with electron-donating groups exhibit higher cytotoxicity than electron-attracting phenols. Additionally, the hydrophobic nature of PCs enhances their ability to induce apoptosis by influencing their interaction with the cell membrane and binding to proteins (caspases) involved in the apoptotic pathway [66]. The ability of kānuka PCs to exhibit antiproliferative activity in tumor cells and non-cytotoxicity in non-transformed cells supports their use as suitable candidates for drug development.
3 Impact of Extraction Efficiency on Kānuka Bioactives
The bioactive composition plays a key role in determining the biological properties of extracts. Although some crude extracts demonstrate synergistic interactions to maximize their biological properties [67], others require isolation of specific compounds to target the desired biological activity. Medicinal plant extracts have been extensively investigated for their beneficial effects in humans. However, many clinical trials have failed owing to their promiscuous activity and cytotoxicity [16]. Therefore, screening of metabolites offers a profound understanding of biochemical composition, allowing for the selection of potential candidates and the removal of undesirable compounds for further application.
Phytochemical analysis is challenging because of its chemical diversity, instability, low concentration, and low solubility [68]. In plants, BCs are often sequestered in specialized secretory structures, including schizogenous passages and central vacuoles of guard and epidermal cells [69]. Extraction is the process of rendering a biomolecule from the storage gland to the medium (solvent). Various conventional and advanced extraction methods have been used to study the plant molecules. Traditional methods, including Soxhlet extraction and maceration, have proven effective for extracting BCs at higher extraction rates. In addition, it aids in a deeper understanding of separation mechanisms and optimization strategies. Novel or advanced extraction methodologies have emerged as promising alternatives, including subcritical water, supercritical fluids, ultrasound, and microwave-assisted methodologies. These methods result in enhanced product yields, suitability for thermolabile compounds, and environment-friendly operations. Furthermore, it provides the opportunity to combine two processes, ultrasound microwave-assisted extraction, to facilitate extraction efficiency [68]. Hence, the chosen extraction should sufficiently disrupt the storage gland to separate or concentrate a variety of biomolecules without altering their biological efficiency from the natural source.
Kānuka's research primarily relies on conventional extraction. Distillation has been predominantly applied to investigate terpenoids, whereas solvent extraction has been employed to study terpenoids and PCs [6-10, 18-20, 23, 28, 30, 37, 50, 54]. Only a few studies have employed the principle of novel extraction, including subcritical water and UAE methodologies, and have reported the accelerated recovery of BCs and their functional properties [11, 12]. Different extractions were used to characterize the kānuka BCs, as presented in Tab. 4. This section further highlights the efficiency and selectivity of the kānuka extraction method.
| Extraction | Compounds | References |
|---|---|---|
| Distillation (ε = 1) | α-Thujene, α-pinene, β-pinene, myrcene, α-terpinene, 1,8-cineole, γ-terpinene, α-terpinolene, linalool, α-terpineol, (E)-carveol, limonene, calamenene, p-cymene, terpinolene, p-cymenene, (E)-linalool oxide, pinocarvone, terpinen-4-ol, myrtenal, (E)-pinocarveol, verbenone, bicyclogermacrene, ledol, spathulenol, viridiflorol, α-gurjunene, ledene, α-muurolene, δ-cadinene, cubebene, p-cymen-8-ol, palustrol, globulol, cis-jasmone, menth-1-ene-4,8-diol, eugenol, viridoflorene, calarene | [10, 18, 22, 23] |
| Solvent extraction | ||
| Methanol (ε = 32.7) | Acyl-phloroglucinols | [8] |
| Hexane (ε = 1.89) | Ficifolidione | [51] |
| Ethyl acetate (ε = 6.02) | Betulinic acid, oleanolic acid, ursolic acid | [10] |
| Methanol (ε = 32.6) | 5,7-Dihydroxy-6-methyl flavanone | |
| Green extraction | ||
| Subcritical water (ε, varies based on temperature) | Gallic acid, catechin, quercetin, syringic acid, 2-hydroxy cinnamic acid, 4-hydroxybenzoic acid, trans-ferulic acid, chlorogenic acid | [11] |
- Note: ε = dielectric constant of solvent at 20 °C.
3.1 Distillation
Distillation is a commonly used method to obtain EOs containing stable oxygenated compounds (alcohols, aldehydes, phenols, esters, ketones, and lactones) and less stable unsaturated compounds (monoterpenes and sesquiterpenes). Volatile components are impermeable to plant membranes. Hence, some physicochemical processes of water, including hydro-diffusion and hydrolysis, are required to liberate the oil from the storage gland. Distillation methods are categorized as water, water steam, and steam distillation [69].
During steam distillation, steam percolates through the plant material, followed by condensation in the flask. Thus, it is suitable for use with water-insoluble volatile compounds. This follows Dalton's Law, in which the total vapor pressure of a system composed of two immiscible solvents equals the sum of their individual vapor pressures. It can separate high-boiling-point EO components at the water temperature [70]. In hydrodistillation, the particles are in direct contact with water. At boiling temperature, water dissolves the oil components and permeates through the membrane by osmosis to reach the outer surface of the matrix. However, prolonged reaction conditions can increase the concentration of alcohols and acids in the product, owing to the chemical modification of oxygenated compounds [69].
The quality and purity of the EOs were determined by evaluating the distribution of organic compounds between the oil and water phases [71]. By optimizing the operational parameters, both distillations yield a high-quality oil. However, losing nonpolar and polar components in the processed distillate still causes significant problems in recovery, requiring additional processing energy and costs. Studies have shown that combining distillation with other extraction methodologies, including adding solvents (co-distillation), can address this issue. In this method, the solvent separates the volatile molecules from the steam and condenses them in a separate flask. However, the choice of solvent, toxicity, and impact on the final products requires more attention. For example, Teixeira et al. [72] demonstrated the effectiveness of solvent-assisted distillation in the recovery of volatile compounds. This study showed improved recovery (65–85 %) from the leaves of Cistus ladanifer L. by adding a solvent (5 % pentane) during extraction, compared to the traditional distillation method (79 %). This technique exploits the difference between polar and nonvolatile molecules, thereby enhancing selectivity and efficiency.
Both distillations were used to investigate the nonpolar compounds of kānuka. Apart from α-pinene, varying proportions of biochemical compounds were observed, owing to the extraction temperature and sample origin. For instance, alcoholic compounds such as p-cymene-8-ol and T-muurolol have been formed by hydrodistillation, owing to the hydrolysis of ester, which is not observed in steam distillation [18].
3.2 Solvent Extraction
Liquid–liquid extraction is the separation or distribution of the solute between two immiscible liquid phases [73, 74]. Solute concentration and extract purification are the primary applications of solvent extraction focuses on solute concentration and extract purification [75]. Hence, it has gained widespread use in various fields, including the screening for secondary metabolites from plants, wastewater treatment, and petrochemical industries. As an emerging technique in drug discovery, solvent-based extraction (bioassay-guided method) has attracted significant interest from researchers. This method utilizes different solvent concentrations to identify active metabolites with intended biological activity [76]. For example, the antiviral bioactive fraction of kānuka was examined using different solvents (ethanol and methanol), and its chemical structure was determined by nuclear magnetic resonance spectroscopy [8].
The efficiency of liquid–liquid extraction depends on many factors, including solvent selection (polarity), extract pH, and temperature [75]. For instance, nonpolar compounds are extracted using solvents such as hexane (ε = 1.89) and ethyl acetate (ε = 6.4), whereas ethanol is more suitable for polar compounds [77]. Interestingly, the synergistic effect of the two miscible solvents enhances the solute yield compared with that of one solvent [73]. Iloki-Assanga et al. [78] demonstrated the influence of solvents on the yield of total phenolic and flavonoid content (TPC and TFC) in Bucida buceras extract using methanol (ε = 32.7) and acetone (ε = 20.7) solvents. They observed that the TPC was higher in the methanol extract, whereas the TFC was higher in the acetone extract. This study highlights the importance of solvent selection as it directly affects the efficiency of yield and specificity in compound separation.
In addition to the dielectric constant, the chosen solvent should provide a sufficient matrix effect to disrupt the cell wall while minimizing the operational hazards associated with volatility and flammability. Additionally, desirable physical properties of solvents, such as low viscosity and density, are advantageous [75, 79]. Commonly used solvents for extraction of plant metabolites include methanol, methylene chloride, and chloroform. These solvents offer distinct advantages because of their ability to penetrate the cell wall and remove extracellular impurities, thus adding significant value to phytochemical extraction [74]. Depending on the nature of the analyte to be extracted, solvent extraction can be categorized as maceration, percolation, infusion, and decoction.
Maceration is a simple extraction technique in which powdered plant material is soaked in a suitable solvent for a long duration with agitation [80]. In the wine industry, prolonged incubation is advantageous for defining the sensory properties of phenolic compounds [81]. According to Lee et al. [82], increasing the extraction temperature and time enhanced the solubility of the solute, increased the yield, and reduced the diffusion coefficient of the solvent. This technique is suitable for isolating low-concentration molecules and exhibits a high matrix effect [79]. Digestion is a modified version of maceration that employs gentle heating to extract poorly soluble PCs without affecting active phytochemicals [83].
Percolation uses fresh solvents continuously to extract BCs [79, 84]. It is commonly used for isolating high-concentration compounds with low solubility in solvents [79], such as oil extracted from seeds. This method is suitable for samples with large particles and produces a high elution rate of the desired metabolites, whereas smaller particles disrupt the preclusion process [74]. The infusion and decoction methods use the same principle as other extractions, where the plant material is soaked in a cold or hot solvent solution [83]. The infusion technique uses volatile compounds, whereas a decoction is applied to heat-stable compounds [85].
Although solvent extraction offers advantages in terms of selectivity and cost-effectiveness, it also poses potential health and safety risks owing to its toxicity and flammability. However, combining solvent extraction with green extraction methodologies, including UAE, offers an improved product yield and quality, reduced energy requirements, and environmental sustainability.
UAE uses ultrasonic waves (ranging from 20 to 100 kHz) to create cavitation bubbles. These bubbles grow and collapse rapidly at a critical point, facilitating chemical changes and causing physical damage to the solid-particle surface. This phenomenon enhances solute mass transfer to a liquid medium [86]. However, optimizing the extraction parameters, such as solvent type and other instrumental parameters, is paramount for achieving the desired product of interest. For instance, determining the frequency range is crucial for maximizing the cavitation energy required to disrupt a solid matrix. In kānuka, UAE has been used to improve the extraction yield with antioxidant and β-secretase inhibitory activities. Thus, optimized conditions have reduced the IC50 required to inhibit the enzyme and scavenge the free radical activity to 14.25 and 3.17 µg mL−1, respectively, compared to Soxhlet extraction, which required 27.95 and 6.55 µg mL−1 [12].
3.3 Subcritical Water Extraction
SWE is a novel green extraction technique that uses the physicochemical properties of water at various temperatures and constant pressures. A higher water temperature disrupts the interaction between the molecule and plant matrices, whereas pressure is maintained in the liquid state. The extraction mechanism is achieved by rapid penetration of water into the plant matrix, followed by disruption of the solute. After separation, the solute diffuses through the intercellular and intracellular fluid materials and is finally eluted into the bulk fluid [87].
Water remains highly polar (ε = 80.1) at ambient temperature, with a long hydrogen-bonding structure. When the temperature increases (100–374 °C) at a constant pressure, the viscosity and dielectric constant of water decrease owing to the breakdown of hydrogen bonds [88]. At a reduced dielectric constant, it behaves as an organic solvent such as methanol (ε = 32.7) and ethanol (ε = 24.5). Thus, it is suitable for separating biomolecules with different polarities [89]. Hence, the optimum extraction temperature for BCs varies depending on the product of interest.
SWE can be performed in two modes. In static mode, the sample matrices are mixed with the solvent, whereas in dynamic mode, the solvent flows through the sample column. Several factors influence the efficiency of SWE, including temperature, time, sample-to-solvent ratio, particle size, and pressure. For example, an investigation of the extraction of BCs from onion skin demonstrated the significant impact of processing variables. They found that (i) increased temperature decreased the concentration of TPC, (ii) alkaline medium (pH) enhanced the antioxidant potential (DHHP), and (iii) smaller particle size enhanced the recovery of total flavonoid compounds [90].
The SWE of kānuka leaves contains a range of phenolic acids and flavonoids with significant antioxidant and antibacterial properties. The operational parameters, including the temperature and solid-solvent ratio, influenced the extraction efficiency. This study has demonstrated the effect of temperature on the extract as follows: (i) color changes from yellow-green to reddish yellow, (ii) increased acidity, (iii) degradation of phenolic compounds, (iv) formation of new compounds at higher temperatures, (v) increased quality (TPC and TFC) and quantity at low temperatures, (vi) reduced phenolic acid content with greater antibacterial and antiproliferative activity at high temperatures, (vii) up to twenty-fold reduced extraction time compared to ethanol extraction, (viii) recovery of a wide range of PCs with different polarities, and (ix) positive correlation between BCs and antioxidant properties [11, 57].
Recently, SWE has gained significant interest for identifying plant metabolites, considering its advantages of range selectivity, cost-effectiveness, water solvent, efficient recovery and processing time, and protection of the structural integrity of compounds and their biological properties [88]. However, the contribution of high temperatures to the recovery process may have some limitations. For instance, SWE hydrolyzes complex molecules into smaller molecules, such as proteins into peptides and amino acids, and polysaccharides into fermentable sugars [91]. Higher temperatures encourage interactions between sugars and amino acids, known as Maillard's reaction, which is characterized by the formation of a brown pigment from polymeric substances called melanoidins in the extract [11]. 5-Hydroxymethyl furfural (5-HMF) is a by-product formed in SWE via Maillard's reaction. Essien et al. [11] observed the formation of 5-HMF in kānuka extract at higher temperatures. Although 5-HMF is considered an indicator of food quality, studies have shown that it exerts dose-dependent cytotoxic, mutagenic, and carcinogenic effects in animal models and cell cultures [92]. Therefore, although SWE aids in the recovery of bioactive molecules, the formation of new products, such as 5-HMF, negatively affects the extract quality. Hence, careful consideration is required when choosing the optimized conditions to minimize the negative effects on the quality of the extract.
4 Conclusion
Natural products are a source of novel metabolites in clinical studies [93]. Taking inspiration from mātauranga Māori, Western science has explored the BCs of kānuka. Research to date has associated the positive antimicrobial, anti-inflammatory, antiproliferative, and antioxidant effects of kānuka with polyphenolic and terpenoid bioactives. In addition, research has emphasized that incorporating green extraction methodologies (SWE) aids in identifying polar and nonpolar metabolites with minimal environmental and human toxicity compared to conventional extraction methodologies. However, the observed biological efficiency was significantly lower than that of the analogs (isolated compounds such as α-pinene) tested. This indicates that despite kānuka containing biologically important compounds as major constituents, the presence of minor compounds in the extract contributes to the observed antagonistic effects. Based on the literature review, we suggest that the isolation of BCs of kānuka may provide more opportunities. We conclude that further insights obtained by transferring the in vitro biological studies reviewed here to in vivo studies offer a route to new drug development and possibly extend to other applications, such as pharmaceuticals and food preservatives.
Acknowledgments
Open access publishing facilitated by The University of Auckland, as part of the Wiley - The University of Auckland agreement via the Council of Australian University Librarians.
Abbreviation
-
- 5-HMF
-
- 5-hydroxymethylfurfural
-
- AD
-
- Alzheimer's disease
-
- BMD
-
- broth microdilution
-
- BCs
-
- bioactive compounds
-
- CDK
-
- cyclin-dependent kinase
-
- DPPH
-
- 2,2-diphenyl-1-picrylhydrazyl assay
-
- DNA
-
- deoxyribonucleic acid
-
- KEO
-
- Kānuka essential oil
-
- FRAP
-
- ferric reducing antioxidant power
-
- IC50
-
- inhibitory concentration required for 50 % inhibition of pathogens
-
- IL-4
-
- interleukin 4
-
- IkBα
-
- inhibitor of NF-kB alpha
-
- LC50
-
- lethal dose required for death of 50 % of organisms
-
- LT50
-
- time required for death of 50 % of organisms
-
- LD50
-
- lethal dose required for death of 50 % of organisms
-
- LB4
-
- leukotriene B4
-
- MBC
-
- minimum bactericidal concentration
-
- MIC
-
- minimum inhibition concentration
-
- MEO
-
- Mānuka essential oil
-
- MFC
-
- minimum fungicidal concentration
-
- mg GAE g−1 dw
-
- milligram gallic acid equivalent per gram of dry weight
-
- mg TE g−1 dw
-
- milligram Trolox equivalent per gram of dry weight
-
- NF-kB
-
- nuclear factor-kappa B
-
- PG
-
- phloroglucinol
-
- PCs
-
- polyphenolic compounds
-
- RIM
-
- radiation-induced mucositis
-
- SWE
-
- subcritical water extraction
-
- TNF-α
-
- tumor necrosis factor-α
-
- TBE
-
- trypan blue exclusion
-
- THP-1
-
- human acute leukemia monocytes
-
- TPC
-
- total phenolic content
-
- TFC
-
- total flavonoid content
-
- ZI
-
- zone of inhibition
Biographies

Indhuja Devadass obtained her Master's in Biotechnology from SRM University, India. Currently, pursuing her Ph.D. in Chemical and Material Engineering at the University of Auckland, New Zealand. Her research explores the potential applications of natural metabolites using green extraction technologies and sustainable resource recovery methods. Indhuja is working on innovative approaches to utilizing bioactive compounds for various applications in pharmaceuticals, food, and environmental sustainability. Her work integrates science and sustainability, driving advancements in green biotechnological solutions.

Simon Swift is Associate Professor of Microbiology and Infectious Disease at Waipapa Taumata Rau the University of Auckland. Swift obtained a BSc(hons) and PhD from Nottingham University, UK specializing in food microbiology. As a postdoctoral scientist he was involved in research on quorum sensing and then microbial iron acquisition. He moved to Auckland in 2001 and has since been involved in research investigating biocides, biofilms, and extracellular vesicles. He is part of a multidisciplinary group investigating the biology and applications of natural products that exhibit a multitude of beneficial traits.

Saeid Baroutian holds the position of Professor within the Department of Chemical & Materials Engineering at the University of Auckland. Additionally, he serves as the Executive Director of the Circular Innovations (CIRCUIT) Research Centre, and Director of the Sustainable Resource Recovery postgraduate program at the University of Auckland. His research focuses on the development and design of innovative and sustainable technologies and methodologies tailored for the circular economy, resource recovery, and waste minimization. Many of his research findings have been embraced by various companies, leading to real-world applications.




