Thermal Conversion of Microalgae into Biochar: A Review on Processes, Properties, and Applications
Abstract
Global energy consumption has drastically increased over the years due to population growth and industrialization. This has prompted an exploration for clean and renewable energy alternatives. Microalgae are widely recognized as a promising third-generation energy source because they have the capability to generate biofuels, including biochar. The utilization of microalgal biomass has been gaining traction because of their advantages, such as fast growth, a high rate of production, and high carbon-fixing efficiency. Thermochemical methods like hydrothermal carbonization, torrefaction, and pyrolysis can be employed to harness energy from microalgae. The different thermochemical methods employed for converting microalgal biomass into biochar have been discussed, as well as the factors affecting these methods. In addition, a dedicated section covered the components and properties of the generated biochar, including its thermal and surface properties. Furthermore, the economic analysis of the production of biochar from microalgae as well as the applications of microalgae-derived biochar were presented and discussed, along with suggestions for further research.
1 Introduction
The rise in population growth and industrialization has substantially increased the need for energy and its utilization. The main source of this energy is fossil fuels, which come at a considerable cost and about whose depletion there are growing concerns. Furthermore, the utilization of fossil fuels leads to the emission of greenhouse gases, which are major contributors to climate change. In particular, the power sector produces significant amounts of CO2, a key greenhouse gas, of which 18.6 % (or almost two billion tons) are emitted into the environment [1]. The hunt for fossil fuel substitutes has been prompted by these harmful environmental consequences. One of the current fossil fuel alternatives that has received the most attention is biomass.
The term “biomass” denotes a varied and complex biopolymer that may constitute a wide variety of compounds, including proteins, lipids, cellulose, hemicellulose, and lignin, among others [2, 3]. It is considered a greener and carbon-neutral source because the quantity of carbon it discharges is equal to the amount absorbed [4]. Furthermore, biomass may be utilized as a renewable energy source to produce biofuel or chemical feedstock, as well as to slow down global warming [5]. Depending on where it comes from, biofuel made from biomass can be categorized as first-, second-, third-, or fourth-generation fuels [6, 7]. Numerous sources, including forests, crops, animal feces, municipal solid waste, industrial waste, agricultural leftovers and crops, and algae, are sources of biomass [8-10]. Food crops serve as the feedstock for first-generation biofuels, whereas non-food crops, including lignocellulosic biomass, are used to make second-generation biofuels [11, 12]. Microalgae are the source of algal biomass used to produce third-generation biofuels [13]. Fourth-generation biofuels focus on genetically modified microalgae to capture substantial amounts of CO2, boost biofuel output, and enhance microalgae adaptability in wastewater through advanced technologies [14].
Microalgae can be developed into biofuels using either biochemical or thermochemical processes. Although thermochemical procedures use the application of heat in an inert or reactive atmosphere, biochemical processes use microbes to transform biomass into biofuel [15]. However, thermochemical processes offer some advantages over biological processes, such as low costs, rapid conversion of biomass, and the lack of time-consuming reaction steps that are inherent to biochemical processes [16, 17]. The thermochemical conversion processes include hydrothermal carbonization (HTC), pyrolysis, torrefaction, gasification, and even direct combustion, and the resulting biofuel products can be solid, liquid, or gaseous fuel.
Biochar produced from the thermochemical conversion of microalgae is a carbon-rich material classified as a solid fuel. This thermochemical process takes place in conditions with low levels of oxygen or no oxygen at all and breaks down the microalgae biomass into volatile gases, liquid bio-oils, and solid biochar [18, 19]. The use of biochar is contingent upon its biomass output and the characteristics of the biochar [20]. Biochar is recognized for its large surface area, porous structure, ability to withstand high temperatures, diverse functional groups, and capacity for ion exchange [21, 22]. Biochar has several environmental and agricultural applications, including sequestration of soil carbon and mitigation of climate change [23, 24], soil amendment [25], the recovery of nutrients from wastewater [26], and as an adsorbent for aqueous pollutant removal [27, 28], among others.
This review provides an overview of the current understanding regarding the thermochemical production of biochar from microalgae. It provides insight into the various parameters influencing the production process and explores the properties, economics, and applications of microalgal biochar.
2 Properties, Cultivation Methods, Advantages, and Disadvantages of Microalgae
Microalgae are highly valued for their diverse composition, which includes lipids, proteins, carbohydrates, and bioactive compounds, making them superior to agricultural crops and other biomass for various applications [29]. The lipid content of microalgae can constitute up to 50 % of their biomass [30], positioning them as a promising feedstock for biodiesel production. Additionally, the negligible or absent lignin content in microalgae offers a distinct advantage over lignocellulosic biomass for biofuel production [31]. Their carbohydrate-rich composition further enhances their potential for the production of bioethanol, biomethane, and biohydrogen [32]. Moreover, with a protein content of up to 70 %, including all essential amino acids, and functional properties, such as foaming, emulsifying, and gelling, microalgae are ideal ingredients for the development of innovative food products [33, 34].
Microalgae biomass, which varies in size, color, and shape, can be cultivated in diverse aquatic environments, such as freshwater, brackish water, saltwater, and hypersaline settings. With a short biomass harvesting cycle of about 1–10 days, microalgae cultivation is significantly faster compared to traditional crops [35, 36]. However, successful cultivation requires careful selection of the appropriate cultivation method. The two most common methods for microalgae cultivation are open culture systems (e.g., raceway ponds, tanks, and open ponds) and closed photo-bioreactors (PBRs) (controlled closed systems employing various bioreactor designs) [37]. Open systems are widely used in industrial-scale microalgae production, accounting for 90 % of annual global microalgae output [38]. Their benefits include low-energy requirements for culture mixing, reduced capital and operational costs, and scalability for large-scale production [37]. Additionally, these systems leverage natural light for photosynthesis, making them simple to design, construct, and operate [39]. However, their exposure to the external environment poses significant challenges. Open systems require large amounts of space for expansion and are susceptible to contamination from sources, such as birds, other microalgae species, bacteria, and grazers, as well as adverse weather conditions [37]. Furthermore, controlling critical growth parameters, such as temperature, carbon dioxide levels, water pH, and dissolved oxygen concentration, is challenging, often leading to suboptimal productivity or even the loss of the target microalgae strain [40]. On the other hand, closed PBRs offer a more controlled environment, addressing many limitations of open systems and ensuring higher quality production [41]. These systems are designed in various configurations, such as column, tubular, and flat plate reactors, to optimize light utilization efficiency, gas–liquid mass transfer, and microalgae harvesting [42]. Tubular PBRs, typically constructed from plastic or glass tubes, are particularly suitable for closed systems [43]. The advantages of PBRs include the ability to maximize productivity in limited space, improved light availability, and significantly reduced contamination risk [37]. Unlike open systems, PBRs allow precise control over nutrients, temperature, pH, and lighting, enabling consistent cultivation of a single-microalgae strain with minimal contamination. They also address challenges like poor CO2 diffusion, water loss, unstable environmental conditions, and high land requirements associated with open systems [38]. However, closed PBRs come with their own drawbacks. These include biofouling, overheating, benthic algae growth, cleaning difficulties, and the accumulation of dissolved oxygen, which can inhibit growth. Most notably, the capital and operational costs of designing and maintaining PBRs are significantly higher compared to open systems [37]. In summary, although open systems are cost-effective and suitable for large-scale applications, closed PBRs provide better control and quality, albeit at higher costs. The choice between the two systems depends on the specific production goals and resource availability.
Microalgae present several advantages for biomass production, particularly when compared to lignocellulosic biomass. First, microalgae exhibit high photosynthetic efficiency and significantly faster growth rates than terrestrial plants [44]. They can be cultivated on non-arable land using various nutrient-rich, low-cost substrates, thereby minimizing competition with traditional agricultural systems [36]. Moreover, microalgae have very short harvesting cycles, ranging from 1 to 10 days, which allows for multiple or even continuous harvests throughout the year [35]. Notably, some species can be harvested twice a day, further enhancing their productivity compared to conventional crops [44]. This impressive production rate positions microalgae as a dominant contributor to the global biomass market, with a projected value of $1.4 billion by 2030 [36]. Microalgae also play a critical role in environmental sustainability. Their negatively charged functional groups make them highly effective for wastewater treatment, particularly in removing nitrogen, phosphorus, and heavy metals [45]. Additionally, microalgae outperform terrestrial plants (e.g., trees) in CO2 capture from industrial exhaust gases, offering a promising solution for reducing greenhouse gas emissions [44]. Nutritionally, microalgae are rich in vitamins (e.g., A, B1, B2, B6, B12, C, and E), minerals (e.g., potassium, iron, magnesium, calcium, and iodine), and proteins containing all essential amino acids, making them a valuable resource for food and feed applications [46]. Furthermore, microalgae are gaining recognition as a promising feedstock for renewable biofuels and bioenergy, such as bioethanol, biohydrogen, biodiesel, and biochar, creating additional revenue streams and attracting significant investments [38, 44]. For example, microalgae species like Chlorella sp., Botryococcus sp., and Chlamydomonas can produce 15–300 times more oil content compared to traditional oilseed crops [35].
Despite these numerous advantages, large-scale production of microalgae remains challenging. Key barriers include the selection of optimal algal strains, development of efficient biomass production methods, and implementation of viable harvesting strategies to recover valuable metabolites [47]. One major challenge is the high cost of harvesting microalgae due to their diluted state, which can account for 20–30 % of the total production cost, making their economic feasibility questionable [48]. Additionally, microalgae cultivation systems, regardless of PBR configuration or production scale, often suffer from significant performance losses due to microbial contamination by bacteria, protozoa, and fungi [49]. Inefficient production systems may also result in undigested nutrients being released into the environment, contributing to aquatic ecosystem disruption and eutrophication. Chanana et al. [50] emphasized that ensuring consistent quality and high yields at a large scale remains a major challenge, particularly when advancing the technology readiness level from laboratory to commercial production. Infrastructure requirements further compound the difficulties of large-scale production. Setting up facilities necessitates substantial investments in materials, equipment, and operational costs, which present a significant economic barrier [51]. Overcoming these challenges will be critical for unlocking the full potential of microalgae as a sustainable and scalable biomass resource.
3 Analysis and Discussion of Bibliometric Findings Regarding Literature on Production of Biochar from Microalgae (2004–2024)
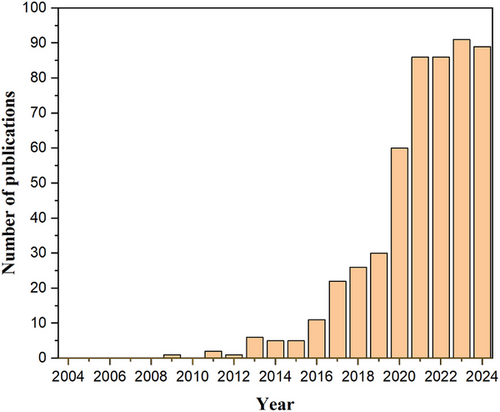
A search for research and publications on the production of biochar from microalgae was conducted using the Web of Science (WOS) database. Articles published between 2004 and December 2024 were retrieved using the query string: (((TS = (microalgae)) and TS = (biochar))). This search incorporated keywords, such as microalgae biochar, microalgal biochar, oxidative torrefaction, biochar and bio-oil, catalytic hydrothermal liquefaction, microalgae and biochar, algal biochar, co-liquefaction, biocrude, and algal biorefinery. A total of 521 articles matching these terms were identified. Metadata for the selected publications was retrieved as of December 15, 2024. The articles were analyzed to assess trends in publication growth over time, and bibliometric analyses were conducted to examine the co-occurrence of author keywords. These analyses were performed using VOSviewer 1.6.17, an open-source science mapping tool. VOSviewer generates distance-based maps where the strength of relationships among items is represented by the proximity of their nodes; shorter distances indicate stronger relationships. This visualization technique aids in identifying clusters of related terms. Each node in the map (e.g., circle or rectangle) represents a specific facet, with node size indicating its significance or influence [52]. Fig. 1 illustrates the annual trends in scientific publications on the production of biochar from microalgae. Notably, research in this area began gaining traction in 2013, with six articles published that year. A significant increase occurred in 2020, with 60 articles published, which is also double the total from 2019. Between 2020 and 2024, the WOS database indexed a cumulative 412 publications, reflecting substantial growth in interest and activity in this field. By December 2024, 89 articles had been published for the year, underscoring a sustained upward trend in research output. This trajectory suggests that the production of biochar from microalgae will continue to attract increased scholarly attention, as evidenced by the marked growth observed in 2023 and 2024.
A co-occurrence network is an effective tool for identifying prominent research areas and uncovering potential future directions [53]. Using VOSviewer, a co-occurrence network map of author keywords was generated by setting a minimum occurrence threshold of 20. Of the 2492 keywords analyzed, 51 met this criterion. In Fig. 2, each circular node represents a keyword, with the node size corresponding to the frequency of its appearance in the articles. The proximity among nodes reflects the degree of relatedness among keywords; closer distances indicate stronger connections. Furthermore, keywords grouped within the same cluster are represented by the same color, signifying a higher likelihood of co-occurrence compared to those in different clusters [39].
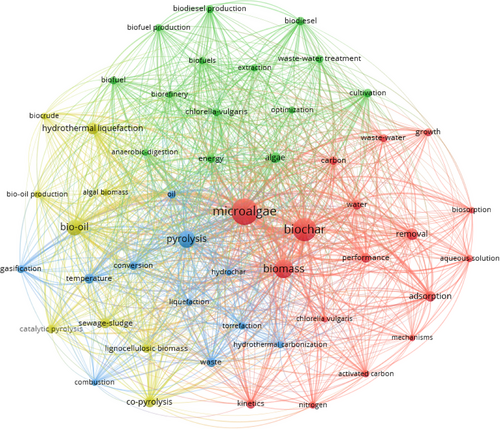
All 51 keywords are organized into four clusters. Cluster 1, highlighted in red and comprising 17 keywords, is the largest. The most frequently occurring keywords are microalgae (336 occurrences) and biochar (263 occurrences). Other notable keywords include adsorption (39 occurrences) and biomass (50 occurrences), reflecting their strong interconnections with other terms. Additional keywords in this cluster include activated carbon, growth, and wastewater, among others. Cluster 2, depicted in green, contains 14 keywords, with algae, cultivation, and energy being the most prominent. Other terms frequently co-occurring in this cluster are biofuel production, biorefinery, biodiesel, extraction, and optimization. These keywords underscore research focusing on bioenergy and the optimization of algae-based biorefinery processes. Cluster 3, depicted in blue, encompasses 11 keywords. This cluster highlights terms associated with thermal conversion processes for producing biochar from microalgae. Key terms include pyrolysis (140 occurrences, 50 links), HTC (27 occurrences, 44 links), gasification (30 occurrences, 39 links), torrefaction (24 occurrences, 46 links), combustion (22 occurrences, 34 links), and conversion (33 occurrences, 43 links). These keywords reflect strong correlations within the domain of thermal transformation technologies. Cluster 4, represented in yellow, consists of nine keywords focusing on sustainable approaches to producing both bio-oil and biochar from the thermal conversion of microalgae. Bio-oil is the most frequently mentioned keyword in this cluster (118 occurrences, 49 links), emphasizing its significance in sustainable biochar production. Other notable terms include catalytic pyrolysis, co-pyrolysis, hydrothermal liquefaction, lignocellulosic biomass, and sewage sludge. Co-pyrolysis (52 occurrences, 43 links) highlights the growing interest in co-thermal processes as a sustainable method for producing biochar. This cluster emphasizes research exploring the intersection of sustainability and economic development.
4 Thermochemical Processes for Microalgae-Derived Biochar Production
Several techniques, including pyrolysis, gasification, HTC, and torrefaction, can be employed to convert microalgae into biochar [54]. HTC and slow pyrolysis are considered the two most effective methods of thermochemical processes for producing biochar. This is because they have a diversity of feedstock adaptability and produce substantial carbon yields [55]. Usually, prior to the carbonization of the microalgae, it is isolated, cultured, collected, and harvested, washed, and freeze-dried, and pulverized to produce a powder, which is then thermochemically modified into biochar [56]. This section discusses the progress that has been made in the usage of various strategies for biochar synthesis from microalgae. Fig. 3 presents an overview of thermochemical processes for producing biochar from microalgae.
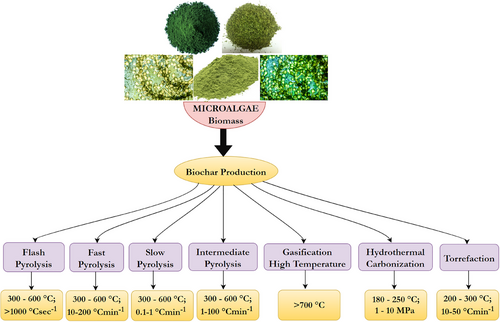
4.1 Pyrolysis
Pyrolysis involves subjecting organic materials like microalgae to high heat in the absence of oxygen, representing a thermochemical process [57, 58]. The pyrolysis of microalgae is regarded as a suitable strategy for sustainable bio-products and bioenergy generation. Under anaerobic circumstances, the microalgae are heated to a high temperature during the pyrolysis process, generally between 300 and 800 °C [59]. As temperature rises, the organic matter in the microalgae undergoes a sequence of chemical and physical transformations, resulting in the creation of biochar [60]. In addition to biochar, the pyrolysis of microalgae also produces liquids and mixtures of gases that can be used as fuels [61]. The gases produced, such as hydrogen, carbon monoxide, and methane, can all be utilized as energy sources for heating and generating electricity [62]. The liquids produced during the pyrolysis of microalgae are typically a mixture of fatty acids, esters, and alcohols, which can be used as a source of biofuels [61].
There are various forms of pyrolysis, each with its own specific properties and uses. Fast pyrolysis, slow pyrolysis, flash pyrolysis, and intermediate pyrolysis are some of the most popular pyrolysis processes [63]. Flash pyrolysis is a form of fast pyrolysis that includes the quick heating of feedstock, often employing hot gases or superheated liquids [64]. This sort of pyrolysis is marked by an extremely small residence period, often lasting milliseconds, paired with a high heating rate (of around 1000–10 000 °C s−1) [65]. Fast pyrolysis is commonly used to make bio-oil, as well as other bio-products like biochar and gases. At high temperatures, generally between 400 and 800 °C, it is a fast thermochemical conversion process [66]. This kind of pyrolysis is marked by a short residence period, often lasting a few seconds, paired with a high heating rate. Bio-oil, a liquid that may be exploited as a source of energy or as a feedstock for chemical manufacturing, is typically created utilizing fast pyrolysis. Intermediate pyrolysis is characterized by a considerable residence duration and heating rate (typically >10 °C min−1) [63]. Intermediate pyrolysis can produce both bio-oil and biochar, as well as other bio-products like gasses. Slow pyrolysis, a low-temperature thermal conversion process, occurs at temperatures between 300 and 600 °C, with a heating rate generally between 0.1 and 2 °C min−1 [67]. This sort of pyrolysis is marked by lengthy residence time, generally lasting several hours, paired with low heating rate. Tab. 1 presents a summary of the process variables for the production of microalgae biochar via pyrolysis.
| Microalgae | Microalgae growth condition | Pyrolysis process condition | Biochar yield | References | ||
|---|---|---|---|---|---|---|
| Peak temperature [°C] | Residence time [min] | Rate of heating [°C min−1] | ||||
| Scenedesmus quadricauda | Cultivated in dairy wastewater under continuous illumination (90–100 µmol photon m−2s−1 at room temperature (22 ± 1 °C) | 750 | 60 | 5 | – | Nakarmi et al. [80] |
| Chlorella sp. | – | 700 | 60 | 10 | 40 | Chang et al. [68] |
| Chlorella sp. | – | 200 | 120 | – | 57.45 | Yang et al. [79] |
| Spirulina sp. | 53.42 | |||||
| Nannochloropsis | Tubular photo-bioreactors (PBRs) operating in continuous mode, with the feed being seawater-containing nutrient source | 500 | – | 15 | 30 | Azizi et al. [81] |
| Isochrysis galbana | 21 | |||||
| Chlorella vulgaris | Batch PBR, with an optimized inoculum size of 0.06 g L−1 and a pH of 6–7, temperature of 26 ± 1 °C with an agitation rate of 300 rpm. The sole carbon source from 2.5, 5.0, and 7.5 % CO2 was continuously supplied to the microalgae culture during cultivation | 500 | 30 | 10 | 26.9 | Yu et al. [82] |
| Chlorella vulgaris | Closed tubular PBR. Grown at room temperature with continuous illumination (338 µmol m−2 s−1) and constant purging with CO2 and air flow rates of 3 and 25 L min−1, respectively. Spectrophotometry of UV–vis with 550 nm wavelength | 500 | – | 5 | 31 | Wang et al. [83] |
| Scenedesmus obliquus | Closed PBRs | 375 | 150 | – | 30–48 | Ferreira et al. [84] |
| Chlorella vulgaris | Open raceway system. Diluted dairy manure stream (45 L) was an input into the system daily | 450 | – | 5 | 42 | Sotoudehniakarani et al. [85] |
| Chlorella vulgaris | PBR (5 L) under continuous white fluorescent light illumination (8000 lx) and at room temperature for 15 days | 300 | 15 | 5 | 53.61 | Koçer et al. [86] |
| Tetraselmis sp. | – | 500 | 60 | 100 | 20 | Aysu et al. [87] |
| Isochrysis sp. | 30 | |||||
| Pavlova sp. | – | 500 | 60 | 100 | 44 | Aysu et al. [88] |
| Spirulina sp. | – | 550 | 60 | 8 | 32 | Chaiwong et al. [89] |
| Scenedesmus dimorphus | – | 500 | – | 40 | 27 | Bordoloi et al. [90] |
| Microcystis sp. | – | 500 | 40 | – | 25.6 | Li et al. [91] |
| Chlorella sp. | Pilot-scale algal biomass production system, integrated with swine-manure wastewater treatment | 500 | – | – | 26–49 | Borges et al. [92] |
| Tetraselmis chui | Cultured indoors under controlled conditions temperature, CO2-enriched air flow (air + 2 % CO2) and light were controlled | 500 | 20 | 10 | 34 | Grierson et al. [93] |
As shown in Tab. 1, Chang et al. [68] demonstrated the application of slow pyrolysis for biochar production using Chlorella sp. as the feedstock. The process was conducted within a temperature range of 300–700 °C, with a gradual increase in temperature at a rate of 10 °C min−1, and maintained for a duration of 60 min. The resultant biochar had high quantities of nitrogen and several inorganic elements like phosphorus, iron, potassium, calcium, and magnesium. Interestingly, the percentage of carbon in the biochar increased from 56.3 at 300 °C to a peak of 66.2 at 500 °C, then it slightly decreased to 65 at 700 °C. Yet, when the temperature was increased, the quantity of hydrogen and nitrogen dropped. Based on these findings, the authors concluded that biochar generated from Chlorella might be employed to make a high-nitrogen, porous, and mineral-rich fertilizer. Another study conducted by Binda et al. [69] examined the slow pyrolysis of Nannochloropsis sp. to generate biochar at 350 °C using a heating rate of 10 °C min−1. Here, it was proposed that the biochar produced might effectively adsorb lead(II) ions from water. The utilization of fast pyrolysis of microalgae has been explored by Azizi et al. [15]. The microalgae Isochrysis galbana, Tetraselmis sp., and Nannochloropsis sp. were pyrolyzed for 500 °C, with the derived biochar having a potential to reduce the level of hydrogen and oxygen content in comparison to raw microalgae. Moreover, the parent biomasses’ surface areas are lesser due to their lower porosity than the biochars making the pyrolysis a potential technique to improve the raw material's morphology and porous structure [15]. Bolognesi et al. [70] revealed that greater biochar yield (80 %) with less ash content than pyrolysis of wastewater treatment plant sludge (with 74 % biochar yield, 30 % ash content) could be achieved when sewage sludge and microalgae are co-pyrolyzed at 350 °C. Likewise, Chen et al. [71] studied the co-pyrolysis of microalgae Dunaliella salina with commonly used plastics like polyethylene terephthalate, polypropylene, polyvinyl chloride, and polystyrene. The investigation indicated that co-pyrolysis led to an increased yield of the desired product while reducing solid waste and the critical energy point. These findings have great significance for co-treatment thermochemical conversion unit design and operation.
Temperature variation directly affects the production and composition of the biochar, hence influencing its characteristics and possible uses. During pyrolysis, higher temperatures generally result in lower biochar yields due to increased carbonization and decomposition of biomass. It was revealed that the yield for two microalgae constantly fell as the temperature increased from 200 to 600°C. Chang et al. [68] noted a comparable finding, asserting that the yield of Chlorella sp. biochar decreased as the temperature increased from 300 to 700 °C. The temperature also affects biochar properties, such as surface area, porosity, and chemical composition. Zhang et al. [26] examined the influence of temperature on the properties of Chlorella pyrenoidosa biochar. They found that with the temperature rising from 650 to 800 °C, there was a reduction in both the surface area and pore diameter of the biochar. This decline implies a contraction of the material's surface with rising temperature, resulting in a reduction of its free surface area [26]. The rate at which heat is applied during thermal conversion process can have an impact on both the reaction kinetics and the distribution of products [72, 73]. Lower rates of heating might allow for more controlled reactions and the production of biochar with desired attributes like increased surface area and pore volume. Conversely, rapid heating rates can result in partial conversion or the generation of volatile chemicals. Koçer et al. [72] conducted research to statistically explore the influence of the heating rate, temperature, and retention time on the yield of biochar obtained from the pyrolysis of Chlorella vulgaris. Their investigation revealed that higher heating rates led to increased biochar yields, whereas the opposite trend was observed with rising temperature and retention time. Katyal et al. [74] showed that the influence of heating rate (5–30°C min−1) on biochar yield obtained from bagasse was evident at carbonization temperatures below 400 °C but not significant at temperatures above 700 °C. The impact of these factors on biochar generation aligns with results observed in prior research involving diverse biomass materials, such as wood, palm kernel shell [75], soybean, peanut shell [76], olive stone [77], and freshwater algae (Spirulina, Spirogyra, and Cladophora) [78], as documented in existing literature. During the pyrolysis of microalgae, residence time greatly effects biochar production. As shown in Tab. 1, research studies demonstrated that employing lower temperatures and longer residence times led to the increased production of microalgae biochar [68, 79].
4.2 Hydrothermal Carbonization (HTC)
HTC is a transformative process that converts wet organic matter into a solid carbon-rich product known as hydrochar [94]. HTC occurs under high temperatures (typically between 180 and 250 °C) and pressures (between 10 and 50 bar) [95], offering an eco-friendly approach to bioenergy and bio-product production. During HTC, wet organic materials like microalgae are subjected to heating in a reactor containing water or a liquid solution, resulting in the formation of solid carbon–rich hydrochar, along with gases and liquid products [96]. The water or liquid solution acts as a reactant and solvent, facilitating the breakdown of the microalgae biomass components. The biomass is subjected to dehydration, decarboxylation, hydrolysis, and polymerization reactions leading to the formation of carbonaceous materials [97].
One notable benefit of HTC is its ability to convert wet organic materials into solid bioenergy products directly, eliminating the requirement for pre-drying, which addresses a significant challenge for numerous feedstocks [98]. This can result in a more efficient and economical process, requiring less energy compared to dry thermal methods. Moreover, HTC has the capability to generate hydrochar with a high carbon content, thus increasing its potential value as a resource [98]. Nevertheless, HTC has some challenges, including the requirement for high-pressure equipment and scalability issues [99]. Additionally, the hydrochar produced through HTC typically possesses a low calorific value, restricting its utility as a fuel source [100]. Addressing these limitations highlights the necessity for ongoing research aimed at refining the HTC process and investigating alternative uses for hydrochar.
The HTC of microalgae has been explored by different researchers. Liu et al. [101] investigated the HTC on Scenedesmus-containing high ash content. The study, conducted in a 350 mL autoclave at three different temperatures (180, 240, and 260 °C) with a retention time of 240 min, demonstrated that hydrochars derived from deashing microalgae (DA) at 220 °C exhibited a carbon content of 51.86 %. This value was 1.15 and 1.33 times higher compared to the carbon content of hydrochars derived from natural microalgae. In another study, Arun et al. [102] explored the HTC method to convert Scenedesmus sp. into biochar. The procedure took place over a duration of 1 h under conditions of 350 °C temperature and 5 MPa pressure. Under these conditions, the biochar yield was 3.4 g/20 g, comprising 8.5 % oxygen, 4.9 % hydrogen, and 85.3 % carbon. The impact of hydrothermal temperature between 180 and 250 °C and retention time of 0.5–4 h was also investigated on the hydrochar's chemical and physical characteristics, produced from C. vulgaris. The findings suggested that increased temperature had a more noticeable effect in reducing hydrochar yield compared to prolonged retention times. Following optimization, the process produced hydrochar with a higher heating value (HHV) of 24.51 kJ g−1 compared to the initial biomass [103]. Some other studies investigating the use of HTC for the production of biochar from microalgae are presented in Tab. 2. Elsewhere, HTC as a first step followed by ethanol extraction holds great potential in generating algal lipids with considerable worth for both biofuel and nutraceutical applications, with about 86 % of the residual fatty acids removed by ethanol, yielding a 74 % overall recovery [104]. As demonstrated by Sztancs et al. [105], application of cofiring ratios of 43.9 % and 53.1 % in hydrochars produced from both catalyzed and noncatalyzed HTC processes is capable of significant reductions in greenhouse gas emissions. Marco et al. [106] conducted a study to investigate the viability of producing carbon-encapsulated iron nanoparticles (ME-nFe) from microalgae through HTC (HTC-LF) of microalgae. When 20 % dilution of the HTC-LF is utilized, there is a promoting microalgae growth without negatively impacting photochemical efficiency. Moreover, HTC can recover organic nitrogen suitable for recycling in microalgae cultivation. Using HTC-derived nitrogen as a source under nitrogen-limited conditions enhances carbohydrate and biomass accumulation in Arthrospira platensis by over 15 % compared to conventional nitrate use [107].
| Microalgae | Microalgae growth condition | Hydrothermal process condition | Biochar yield | References | ||
|---|---|---|---|---|---|---|
| Peak temperature [°C] | Residence time [min] | Rate of heating [°C min−1] | ||||
| Chlorella vulgaris | – | 220 | 60 | 5 | 43.4 | Jabeen et al. [113] |
| Chlorella vulgaris | Chlorella vulgaris microalgae were cultivated using a mixotrophic culture medium comprising a varying concentration of wastewater and BG-11 medium in a photo-bioreactor (PBR) having a volume of 1 L, a light intensity of 4000 lx for 12 h, a CO2 flow rate of 0.2 vvm, and a temperature of 27 ± 3 °C with a neutral pH | 350 | 60 | 20 | – | Thillainayagam et al. [114] |
| Spirulina platensis | Closed system and freeze-dried | 190 | 60 | 7 | – | Zhao et al. [115] |
| Chlorella vulgaris | – | 180 | 30 | – | 74.53 | Lee et al. [116] |
| Scenedesmus sp. | Hybrid loop airlift PBR (HLALPBR) using 5 L of domestic wastewater. Temperature of 30 ± 2 °C and light intensity of 4000 lx for a time of 16 days | 350 | 60 | – | 3.4 g/20 g of feedstock | Arun et al. [102] |
| Chlorella sp. | PBR (water temperature was maintained at 25 ± 1 °C, and the light quantity was 100 mmol m−2 s−1 with 12 h of light–dark intervals). The hydraulic retention time (HRT) of PBR was 5 days | 180 | 30 | – | 98.23 | Lee et al. [111] |
| Nannochloropsis | – | 160 | 30 | – | 65.86 | Mata et al. [117] |
| Spirulina platensis | – | 180 | 30 | 4 | 45.7 | Zhang et al. [118] |
| Chlorella vulgaris | Cultured in two raceway pond. The flue gases containing approximately 10 % CO2 (v/v) were added to maintain the culture pH of 8.0. Temperature and light were not controlled and reflected those naturally available in this area. The raceway ponds were inoculated with an inoculum/wastewater ratio of 240 | 180 | 60 | – | 55 | Benavente et al. [112] |
| Hippeastrum reticulatum | Cultivated in the secondary effluent of treated wastewater | 150 | 60 | – | 0.72 g/feed mass | Park et al. [119] |
| Spirulina maxima | – | 175 | 30 | – | 49.3 | Broch et al. [120] |
| Chlorella vulgaris | – | 250 | 60 | 10 | 15 | Ekpo et al. [121] |
| Picochlorum oculatum | Grown in an outdoor novel horizontal bioreactor | 180 | 120 | 5 | 36 | Tsarpali et al. [122] |
| Arthrospira platensis | – | 190 | 120 | 6 | 36.7 | Yao et al. [123] |
| Microcystis sp. | Cultured in poultry farm wastewater with an initial concentration of 41.3 mg P kg−1 constant aeration (4 mL s−1), photoperiod of 14 h:10 h light:dark cycles, at a controlled temperature of 25 ± 1 °C under cool white fluorescent light of 10 000 lx intensity | 200 | 120 | 3 | 58.8 | Chu et al. [124] |
| Chlorella vulgaris | 49.8 | |||||
| Chlorella vulgaris | Maintained in 2 L borosilicate bioreactors using sterilized medium, 3N-BBMV. The operational conditions were as follows: constant aeration using 2.5 % CO2 at 0.2 vvm, a photoperiod of 14:10 light:dark cycles, 150 µmol m−2 s−1 of luminance, and a temperature of 25 ± 1 °C | 260 | 60 | – | – | Chu et al. [125] |
Temperature plays a crucial role in HTC as it determines the reaction kinetics and product distribution. Optimal temperatures for HTC typically range from 180 to 250 °C, although variations may occur depending on the specific microalgae feedstock and desired biochar properties. Studies show that higher reaction temperatures lead to decreased volatile matter content but increased fixed carbon and HHV of hydrochars [108, 109]. The effects of temperature on the yield and the properties of the hydrochar obtained from the HTC of seaweed (macroalgae) were studied by Patel et al. [110]. The hydrochar generated at 220 °C exhibited the highest carbon content (48.5 %) and heating value (18.93 MJ kg−1). Additionally, both the energy density and carbon to nitrogen (C/N) ratio in the hydrochar substantially rose in comparison to the raw seaweed. The rate at which heat is applied has a considerable effect on the generation of biochar from the HTC process of microalgae. Several research studies have indicated that altering the carbonization temperatures, residence periods, and heating rates during the process impacts the yield of the hydrochar, as shown in Tab. 2 [111, 112].
4.3 Torrefaction
Torrefaction is defined as one of the thermochemical processes that is used to convert organic matter, such as microalgae, into a solid carbon product known as torrefied biomass [126]. The process involves exposing the organic material to moderate temperatures (between 200 and 300 °C) in a low-oxygen environment, causing dehydration, decarboxylation, and condensation of the organic matter into a solid, carbon-rich substance [127]. In the case of microalgae, torrefaction can transform the algae into a solid, dense, and homogeneous carbon-rich material that is stable compared to the original algae [128]. The process also reduces the moisture content of the microalgae, making it easier to handle and transport. There are several types of torrefaction that can be applied to microalgae, including wet and dry torrefaction.
The traditional method of torrefaction, known as dry torrefaction, involves heating microalgae in an environment with restricted oxygen, without the presence of a liquid phase [129]. The process decreases the moisture content of the microalgae, yielding a dry, solid, carbon-rich substance. Dry torrefaction is a straightforward process that may be conducted utilizing conventional thermal treatment technologies, such as rotary kilns or fluidized bed reactors [130]. In wet torrefaction, microalgae are heated in the presence of a liquid phase. This approach allows for efficient heat utilization, with water acting as a medium for heat transfer, thereby reducing the thermal resistance of the organic matter [131]. Additionally, wet torrefaction produces a carbon-rich material with hydrophobic characteristics, rendering it less prone to degradation when compared to untreated microalgae [130]. HTC and wet torrefaction are related [132] in that it entails heating wet organic matter in the presence of a liquid phase. However, there are some key differences between the two processes, including the pressure and temperature conditions used, the products produced, and the applications of the resulting material [133]. Both dry and wet torrefaction processes have their advantages and disadvantages. Dry torrefaction, being a straightforward process, can be executed using conventional thermal treatment technologies, making it well suited for large-scale applications [134]. On the other hand, wet torrefaction is more energy-efficient and yields a carbon-rich material that is hydrophobic and less susceptible to degradation, making it more suitable for applications in agriculture and soil improvement [135].
As shown in Tab. 3, the investigation, concentrating on two microalgae strains, C. vulgaris ESP-31 and Chlorella sp. GD, demonstrated that wet torrefaction conditions led to the highest biochar production. Specifically, at a temperature of 160 °C and a brief holding time of 5 min, biochar yields of 54.5 % and 74.6 % were achieved for C. vulgaris ESP-31 and Chlorella sp. GD, respectively. This method enhanced the fuel properties and added value to the environment [136]. Viegas et al. [137] assessed the co-torrefaction of lignocellulosic biomass and microalgae and analyzed its energy efficiency. Results showed that lower temperatures had higher energy efficiency (92.6 %), but this decreased significantly as the feed's mixture moisture content increased (ranging from 16.9 % to 57.3 % for 70 % moisture). Under optimal conditions, the biochar production reached its highest point at 76.5 %, along with a heating value of 17.4 MJ kg−1. In a separate study, Yu et al. [138] investigated the adsorption characteristics of methylene blue (MB) and Congo red (CR) dye types on biochar derived from wet-torrefied Chlorella sp. microalgae. The focus of their investigation was on the biochar's ability to adsorb cationic and anionic dyes. The wet-torrefied biochar of microalgae displayed microporous characteristics, with pore sizes smaller than 2 nm, indicating its potential as an effective adsorbent for eliminating hazardous dyes. Compared to similar biochar adsorbents, MB and CR exhibited maximum adsorption capacities of 113.00 and 164.35 mg g−1, respectively, with the percentage removal efficiency ranging from 26.32 % to 89.78 % and 54.72 % to 97.10 %. Similar to coal fuel, torrefied microalgae and other biomass exhibit similar HHVs as well as H/C and O/C atomic ratios, indicating their potential to replace fossil fuels for power generation in power plants. This is because the torrefied microalgae worked on possesses a higher HHV, greater carbon content, but reduced atomic ratio [139].
| Microalgae | Microalgae growth condition | Torrefaction process condition | Biochar yield | References | ||
|---|---|---|---|---|---|---|
| Peak temperature [°C] | Residence time [min] | Rate of heating [°C min−1] | ||||
| Chlorella vulgaris ESP-31 | An open pond | 160 | 10 | – | 47.25 | Gan et al. [142] |
| Chlorella vulgaris FSP-E | An open pond | 160 | 10 | – | 66.90 | Gan et al. [142] |
| Chlorella sorokiniana MB-1-M12 | Two-stage cultivation strategy | 200 | 10.6 | – | 92.15 | Felix et al. [143] |
| Nannochloropsis oceanica | – | 300 | 60 | – | 80 | Zhang et al. [144] |
| Chlorella vulgaris ESP-3 | – | 160 | 10 | – | 54.5 | Yu et al. [136] |
| Chlorella sp. GD | – | 160 | 10 | – | 74.6 | Yu et al. [136] |
| Nannochloropsis Oceanica | – | 200 | 15 | – | 96.42 | Zhang et al. [141] |
| Chlorella sp. | – | 200 | 15 | – | 93.26 | Zhang et al. [141] |
| Chlorella vulgaris ESP-31 | Grown outdoors in a 50 L vertical tubular photo-bioreactor | 200 | 15 | – | 96.8 | Chen et al. [145] |
| Chlamydomonas sp. JSC4 | – | 200 | 30 | – | 93.9 | Chen et al. [146] |
| Scenedesmus obliquus CNW-N | Isolated from freshwater consumes CO2 efficiently while it grows | 200 | 60 | 40 | 86.37 | Chen et al. [147] |
| Chlamydomonas sp. JSC4 | Isolated from freshwater | 200 | 60 | – | 86.87 | Chen et al. [148] |
| Chlorella sorokiniana CY | Isolated from freshwater | 200 | 60 | – | 85.76 | Chen et al. [148] |
The extent of thermal decomposition and carbonization during torrefaction is influenced by temperature alteration. Viegas et al. [140] investigated the co-torrefaction of lignocellulosic biomass and microalgae to produce biochar. They found that the highest biochar yield, reaching 76.5 %, was achieved at 250 °C, accompanied by a favorable calorific value of 17.4 MJ kg−1. The optimal torrefaction temperatures for microalgae biomass typically range from 200 to 300 °C, depending on the desired biochar properties and application requirements. The biochar yield and characteristics are affected by the heating rates used during the torrefaction process of microalgae biomass. Higher heating rates can lead to enhanced activation energies and combustion performance of the biochar [72]. Additionally, the torrefaction process, conducted at different temperatures and heating rates, impacts the yield of biochar, with optimal conditions differing across various microalgae species as shown in Tab. 3. The period of time that biomass remains at the reaction temperature (residence time) determines the extent of biomass decomposition and biochar production. Longer residence times often result in higher carbonization and higher biochar yield, as more time is available for heat reactions to occur. However, extremely extended residence durations may lead to over-charred or deteriorated biochar. For instance, increasing the residence time in the co-torrefaction of lignocellulosic and microalgae biomass improves both the biochar yield [141].
5 Production of Biochar from Microalgae
5.1 Compositions of Microalgal Biochar: Proximate and Ultimate Analyses
Proximate analysis provides valuable information about crucial characteristics, such as moisture content, fixed carbon, volatile matter, and ash content of a given biochar sample, whereas ultimate analysis reveals the organic and inorganic constituents present in the biochar. The data regarding the proximate and ultimate compositions of microalgal biochar from various sources are presented in Tab. 4. Moisture content reduces the combustibility of solid fuel and lowers combustion chamber temperature. The majority of the biochar has a very low moisture content (1–13.2 wt %). This is a desirable property because after thermal pre-treatment, low moisture content samples are easier to handle and keep for longer periods of time [149]. Biochar derived from micro- and macroalgae generally demonstrates lower levels of fixed carbon and volatile matter. Although some studies presented in Tab 4 indicate notable levels of fixed carbon and volatile matter, biochar derived from microalgae generally demonstrates lower yields compared to biochar from lignocellulosic biomass. With increasing thermochemical processing temperature, there is a rise in the fixed carbon content and a decrease in volatile matter [150, 151]. The non-volatile and non-combustible components of a material are measured by the ash content [152]. High ash concentration, however, is not good for biochar because it reduces its qualities as a soil supplement, promotes erosion, and contains elements like chlorine and sulfur that have negative environmental consequences [1, 6]. Pre-treatment of microalgal biomass with water or washing with acid before conversion can help minimize the ash composition of the biochar product [1, 153].
| Microalgae | Proximate analysis [wt %] | Ultimate analysis [wt %] | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Moisture | Fixed carbon | Volatile matter | Ash | C | H | N | S | O | ||
| Nannochloropsis | – | 21.25 | 3.55 | 74.94 | 72.39 | 4.47 | 11.46 | – | 11.68 | Azizi et al. [15] |
| Tetraselmis | – | 30.44 | 5.98 | 62.49 | 71.78 | 3.66 | 10.53 | – | 14.03 | Azizi et al. [15] |
| Isochrysis galbana | – | 26.70 | 4.16 | 68.83 | 72.45 | 3.38 | 11.18 | – | 12.99 | Azizi et al. [15] |
| Chlorella sp. | – | – | – | 25.57 | 56.25 | 5.78 | 12.40 | – | 20.90 | Chang et al. [68] |
| Chlorella vulgaris | 3.81 | 65.34 | 17.40 | 13.45 | 61.32 | 3.55 | 9.76 | 0.02 | 11.92 | Yu et al. [82] |
| Chlorella vulgaris ESP-31 | 1.76 | 15.64 | 77.88 | 4.72 | 57.40 | 8.85 | 3.79 | – | 29.96 | Yu et al. [136] |
| Chlorella sp. GD | 2.98 | 16.09 | 74.65 | 6.28 | 54.09 | 8.00 | 10.49 | – | 27.42 | Yu et al. [136] |
| Chlorella vulgaris | 5.0 | 25.1 | 62.6 | 12.3 | 70.1 | 6.7 | 12.1 | 0.5 | 10.6 | Viegas et al. [140] |
| Chlorella sp. | 2.0 | – | 32.4 | 12.8 | 35.0 | 9.2 | 3.0 | 2.0 | 2.5 | Ashokkumar et al. [158] |
| Chlorella vulgaris | 1.5 | 35.6 | 54.4 | 8.5 | 32.5 | – | 5.65 | – | – | Sotoudehniakarani et al. [85] |
| Chlorella vulgaris | 5.8 | 22.9 | 11.2 | 60.1 | 28.3 | – | 4.12 | – | – | Sotoudehniakarani et al. [85] |
| Chlorella vulgaris | 3.38 | 54.20 | 23.46 | 19.98 | 61.96 | 3.87 | 9.43 | – | 4.78 | Wang et al. [83] |
| Spirulina sp. | – | 69.98 | 19.59 | 10.43 | 66.60 | 1.30 | 9.42 | – | 12.25 | Choi et al. [159] |
| Spirulina sp. | 1.4 | – | 58.4 | 40.2 | 70.35 | 4.48 | 9.19 | – | 15.98 | Leng et al. [160] |
| Microalgae consortium | 6.72 | 19.6 | 64.05 | 9.63 | 51.33 | 6.68 | 4.46 | 0.34 | 37.19 | Saiyud et al. [161] |
The biochar's chemical composition is influenced by factors, such as the conversion temperature, residence time, and the type of biomass used. Throughout the biomass conversion process into biochar, mechanisms like devolatilization, decarboxylation, and dehydration lead to a decrease in hydrogen and oxygen content. On the other hand, elevated temperatures and extended residence times lead to a higher carbon content in the biochar [154]. Although biochar made from lignocellulosic biomass often has a larger carbon content than biochar made from microalgal biomass, the amount of carbon reported in the majority of the research under evaluation is still rather high and on par with other biochars. Three different microalgae (Nannochloropsis, Tetraselmis, and I. galbana) were found to contain over 70 % of carbon, according to Azizi et al. [15]. This exceeds the carbon content found in biochar derived from lignocellulosic sources like sugarcane bagasse (66.38 %) [155], rice husk (66.14 %) [156], and African balsam leaves (64.72 %) [22]. Microalgal biochar has a substantially higher nitrogen concentration than other types of biochar because it contains a lot of proteins [68, 82, 83, 140, 157]. This higher nitrogen content enhances its soil amendment property.
The presence of hydrocarbons and organic carbons is also important for enhancing soil properties through biochar. Higher H/C and O/C values suggest a relatively higher presence of aliphatic and oxidized compounds, whereas lower values indicate a higher concentration of aromatic and reduced compounds [162, 163]. Additionally, the cation exchange capacity (CEC) [164] of biochar, which gauges its capability to bind cation nutrients, is another crucial chemical characteristic. Biochar with a high CEC can aid in decreasing nutrient leaching from the soil [165]. Studies have indicated that microalgal biochar tends to possess a higher CEC compared to biochar derived from other sources, such as orange pomace and vine pruning [151]. In general, microalgal biochar tends to have lower carbon content, surface area, and cation exchange capacities compared to biochar obtained from lignocellulosic biomass. Nevertheless, it generally exhibits higher pH, ash, nitrogen, and extractable inorganic nutrient contents [166, 167].
5.2 Microalgae Biochar Properties
5.2.1 Surface Properties
The physical characteristics of biochar, including its surface area, surface charge, porosity, particle size, pore volume, and water-holding capacity, are fundamental factors that influence its effectiveness as both an adsorbent and a soil amender [18, 168]. Various analytical techniques, such as Brunauer–Emmett–Teller (BET) technique for accessing surface area and transmission electron microscopy and scanning electron microscopy (SEM) for morphology and particle size evaluation, are utilized to assess these properties. Research has shown that with rising pyrolytic temperatures, there is typically an increase in both the surface area and pore volume of biochar. For instance, Choi et al. [159] noted a marginal increase in the surface area of biochar produced from Spirulina sp., ranging from 0.31 to 2.63 m2 g−1, with the increase in pyrolytic temperature from 350 to 750 °C (shown in Tab. 4). It is worth mentioning that in contrast to biochar obtained from lignocellulosic materials, microalgal biochar generally demonstrates a reduced surface area.
Zheng et al. [56] produced biochar from the pyrolysis of Chlamydomonas sp. with a surface area of 15.03 m2 g−1, surpassing the surface area of the raw microalgal biomass (4.9 m2 g−1). However, this surface area was significantly lower compared to biochars derived from other sources, such as orange peel (104.37 m2 g−1) [169] and bamboo stem (327 m2 g−1) [170]. Moreover, doping of microalgae biochar with nitrogen can increase its surface area [171], as can iron doping on the biochar [172] and salt modification of the biochar [173]. Shi et al. [173] discovered that with KOH modification in a 1:6 ratio, the surface area of biochar originating from Chlorella sp. and Spirulina sp. increased from 5.0 to 311.7 m2 g−1 and 5.7 to 381.9 m2 g−1, respectively. Tab. 5 summarizes the surface attributes of biochar derived from microalgae. In the work of Yang et al. [174], the biochar obtained from the pyrolysis of residual Spirulina sp. algae after lipid extraction demonstrated an increase in specific surface area and average pore size as the pyrolysis temperature was raised from 400 to 600 °C. The specific surface area rose from 6.49 to 7.89 m2 g−1, whereas the average pore size expanded from 34.73 to 53.68 nm. A similar trend was observed in the specific surface area and average pore size of the resulting biochars when the pyrolysis temperature of residual Spirulina sp. algae was varied from 200 to 550 °C [175]. In this study, there was a slight increase in both the specific surface area and average pore size of the biochar, moving from 4.4370 m2 g−1 and 43.1990 nm to 7.3610 m2 g−1 and 54.7280 nm, respectively. This enhancement is attributed to the development of exposed pores as a result of volatiles released within the biochar matrix during the pyrolysis process. As depicted in Tab. 5, Leng et al. [160] found the surface area of Spirulina sp.–derived biochar to be 1.56 m2 g−1, with an average pore diameter of 32.4 nm. Following a convenient classification suggested by the International Union of Pure and Applied Chemistry [176], pore sizes are broadly divided into three groups: micro-pores (below 2.0 nm), mesopores (between 2.0 and 50 nm), and macropores (above 50 nm). Hence, the biochar produced in their study was identified as predominantly mesoporous material, as its average pore diameter fell within the range of 2.0 and 50 nm.
| Microalgae | Temperature [°C] | Surface area [m2 g−1] | Pore volume [cm3 g−1] | Pore diameter [nm] | References |
|---|---|---|---|---|---|
| Coelastrum sp. | 600 | 2.12 | 0.006 | 15.25 | Zheng et al. [56] |
| Chlorella sp. | 600 | 6.16 | <0.001 | 151.38 | |
| Chlamydomonas sp. | 600 | 15.03 | 0.001 | 152.44 | Zheng et al. [56] |
| Scenedesmus quadricauda | 750 | 35.66 | 0.062 | – | Nakarmi et al. [179] |
| Chlorella sp. | 400 | 5.40 | 0.08 | 46.41 | Zheng et al. [56], Yang et al. [174] |
| Chlorella sp. | 600 | 4.05 | 0.07 | 45.63 | Yang et al. [174] |
| Spirulina sp. | 400 | 6.46 | 0.07 | 34.73 | Yang et al. [174] |
| Spirulina sp. | 600 | 7.89 | 0.07 | 53.68 | Yang et al. [174] |
| Chlorella sp. | 550 | 5.70 | 0.008 | 46.41 | Yang et al. [175] |
| Spirulina sp. | 550 | 7.36 | 0.007 | 54.73 | Yang et al. [175] |
| Scenedesmus sp. | 350 | 117 | 0.01 | 10 µm | Arun et al. [102] |
| Chlorella vulgaris | 450 | 2.90 | – | – | Sotoudehniakarani et al. [85] |
| Chlorella vulgaris | 500 | 2.40 | – | – | Wang et al. [83] |
| Spirulina sp. | 350 | 0.31 | – | – | Choi et al. [159] |
| Spirulina sp. | 750 | 2.63 | – | – | Choi et al. [159] |
| Spirulina sp. | 350 | 1.56 | 0.014 | 32.4 | Leng et al. [160] |
| Microalgae consortium | 400 | 32.66 | 0.002 | 13.7 | Saiyud et al. [161] |
| Chlorella sp. | 800 | 5.00 | 0.01 | 3.00 | Shi et al. [173] |
| Spirulina sp. | 800 | 5.70 | 0.01 | 5.23 | Shi et al. [173] |
| Chlorella vulgaris | 850 | 15.45 | 0.12 | 6.50 | Nejati et al. [180] |
| Chlorella sp. GD | 170 | 2.66 | 0.0004 | 0.66 | Yu et al. [178] |
In a separate investigation, Shi et al. [173] observed that biochars derived from the pyrolysis of Chlorella sp. (C8) and Spirulina sp. (S8) at 800 °C exhibited limited total pore volume and BET surface area. As shown in Tab. 4, the BET-specific surface areas of C8 and S8 were found to be 5.0 and 5.7 m2 g−1, respectively, with identical pore volumes of 0.01 cm3 g−1. However, when these microalgae biochars were subjected to modification, their pore structures improved significantly, resulting in larger total pore volumes and BET surface areas. Upon doping the biochars C8 and S8 with urea, denoted as CN and SN, respectively, the BET surface areas of CN and SN were found to be two to three times greater than those of the unmodified biochars (C8 and S8). Further modification with KOH, by varying the KOH to CN/SN mass ratios from 0 to 2, led to a remarkable increase in the total pore volumes and BET surface areas of these microalgae biochars. The highest BET surface areas were observed at KOH to CN/SN mass ratios of 2, resulting in CNK-2 (422.6 m2 g−1) and SNK-2 (404.8 m2 g−1) for Chlorella and Spirulina, respectively. In comparison, the BET surface area of CNK-2 was 84.5 times higher than that of C8, and the BET surface areas of SNK-2 were about 71 times greater than S8. These findings underscore the significant enhancement in the pore structure of the produced biochars through combined modification, involving urea doping and KOH activation.
SEM spectroscopy is commonly employed to analyze the structure of biochar. SEM analysis is important in determining the biochar's adsorption potential as it shows the degree of pores present in the biochar where the pollutants can cross-link [28, 177]. Arun et al. [102] discovered that the morphology of Scenedesmus sp. is made up of small cracks and pores of size 10 µm. The formation of these void pores was a result of the degradation of moisture impurities during the HTC process. As shown in Tab. 4, the surface area of the microalgae-derived biochar was found to be 117 m2 g−1. Yu et al. [178] employed field emission SEM to analyze the surface structure of biochar obtained from wet torrefaction of Chlorella sp. GD. The examination revealed an irregularly shaped porous structure with rough surfaces. These features are instrumental in providing the necessary active binding sites essential for the adsorption of CR dyes.
5.2.2 Functional Groups
Fourier transform infrared (FTIR) spectroscopy is applied to detect the functional groups present in biochar. This analytical method enables the comparison of chemical constituents among biochar produced under various conditions and the distinctions between raw microalgae and the resultant biochar [181]. Koçer et al. [86] documented several peaks in biochar derived from pyrolysis of C. vulgaris, including absorption bands at 2920 and 1535 cm−1 corresponding to C─H and C═C stretching vibrations, respectively. Additionally, peaks at 1629 and 1031 cm−1 confirmed the presence of protein and carbohydrates in the sample, respectively. Notably, the high pyrolytic temperature (700 °C) resulted in significant differences between the two samples when compared to the FTIR spectrum of the raw microalgae. Increasing the temperature resulted in a reduction in specific peaks, including those at 3273 cm−1 (attributed to O─H stretching) and 2920 cm−1 (associated with C─H stretching). At elevated pyrolytic temperatures, the transition from aliphatic to aromatic structures, the creation of aromatic rings, and hydrogen deformation are known to take place [182]. At lower temperatures, biochar tends to contain a greater amount of volatile carbon, making it more beneficial for improving soil nutrient content [150, 183]. Yu et al. [178] conducted research into how pyrolysis affects the structure and composition of functional groups present in microalgae. They observed significant changes in the FTIR spectra of both C. vulgaris FSP-E and its resulting biochar. Unlike the raw feedstock, the biochar spectra exhibited the disappearance of certain peaks and the emergence of new ones, indicating the release of volatiles and the cleavage of chemical bonds during pyrolysis. The application of high temperatures during pyrolysis resulted in the removal of functional groups like CC, C═C, C─O, and N─H from the microalgal biochar. However, the structure also displayed the presence of new aromatic C─H and alkene C═C functional groups.
5.2.3 Thermal Properties
Thermogravimetric analysis (TGA) and differential thermal analysis (DTA) are employed to examine the thermal behavior of biochar. The TGA curve illustrates the relationship between weight loss and temperature, revealing processes, such as dehydration, decomposition, and oxidation, whereas the DTA curve illustrates how weight change occurs in response to temperature fluctuations over time [161, 184]. The degradation of microalgal biochar can occur in steps as the temperature increases. The elimination of moisture and quick-evaporating volatile chemicals from the biochar mostly causes the first phase to take place, whereas the other steps are dependent on the major compositions of the microalgae source, such as lipids, proteins, and carbohydrates [167]. The thermogravimetric characterization of the biochar in the study of Spirulina sp.–derived biochar, according to Leng et al. [160], revealed that the less volatile organics, such as fixed carbons and non-volatile ash like metal compounds, from the raw microalgal biomass persisted in the biochar. The decomposition of the less volatile organics caused the biochar to lose around 10 % of its weight between 400 and 600 °C, where it remained thermally stable up to that point. After 800 °C, approximately 77 % of the biochar was still retained in the residue.
5.3 Applications of Microalgae Biochar
5.3.1 Sequestration of Carbon Dioxide (CO2)
Due to its adverse environmental impacts, the reduction of CO2 emissions is currently a heavily researched area. CO2 is the primary gas released into the atmosphere during the combustion of fossil fuels. Distillate fuel emits carbon at a rate of 21.95 kg per gigajoule (kg C GJ−1), whereas natural gas emits 14.54 kg C GJ−1. Liquefied petroleum gas contributes 18.69 kg C GJ−1 of total CO2 emissions, and coal emits 25.16 kg C GJ−1 [150]. The utilization of biochar for CO2 sequestration is gaining momentum due to its resistance to degradation and its ability to retain CO2 for extended periods, sometimes hundreds of years [185]. The process is intriguing and straightforward. During photosynthesis, plants absorb atmospheric carbon, storing it within their structures throughout their lifespan. However, upon the plant's decomposition, this carbon is released back into the atmosphere, completing the carbon cycle. By converting carbon into a stable structure that resists degradation, the production of biochar disrupts the carbon cycle, preventing greenhouse gases from returning to the atmosphere [186, 187]. Therefore, large-scale biochar synthesis could potentially impact the atmospheric carbon balance by reducing carbon concentrations [186]. In research by Yu et al. [82], C. vulgaris FSP-E was grown in PBRs using different CO2 gas concentrations as the primary carbon source. With a yield of 26.9 %, the biomass underwent pyrolysis to generate biochar. The resulting biochar showcased an alkaline pH and favorable O/C and H/C ratios, essential for carbon sequestration.
5.3.2 Soil Fertilizer
Microalgal biochar has also been applied to soils as fertilizer. How biochar enhances the physical characteristics of soils depends in large part on the characteristics of the source soil and the feedstock used to make the biochar [188]. Biochar needs to have a dry mass carbon concentration of more than 50 %, a nitrogen and phosphorus content between 1 % and 45 %, a pH under 10, and a surface area of more than 150 m2 g−1 for good agricultural application [189, 190]. In a pot experiment, Arun et al. [102] employed biochar made from Scenedesmus sp., which had previously been used to recover phosphorus, as fertilizer. To prevent their leaching, phosphate- and ammonia-solubilizing bacteria were first used to activate the used biochar. The cultivated plant had higher potential as a fertilizer compared to commercial di-ammonium phosphate, achieving an average height of 22 cm and possessing a chlorophyll content of 24 mg g−1.
5.3.3 Catalyst
The utilization of biochar as an economical carbonaceous catalytic support is plausible. This is due to biochar's abundance of functional groups and pores, both of which can serve as catalysts [191]. The incorporation of biochar produced via the pyrolysis of biomass has been discovered to dramatically speed up the generation of hydrocarbons in bio-oil [192] and the transformation of tar into gaseous products [193]. To improve its ability to act as a catalyst, biochar can also be easily functionalized using groups like ─SO3H [194]. Nejati et al. [180] demonstrated this characteristic by utilizing biochars originating from the pyrolysis of C. vulgaris, both in their raw state and after surface modification, as catalysts to enhance bio-products derived from the same microalgae biomass. Their analysis revealed that the biochar catalyst boosted energy recovery from 58.51 % to 64.46 %.
5.3.4 Low-Cost Adsorbent
The porous nature, affordability, and usability of microalgal biochar have garnered research interest in its potential application as an adsorbent for use in pollution remediation. Biochar derived from the thermal conversion of microalgae biomass has demonstrated effectiveness in adsorbing both organic and inorganic pollutants. A crucial factor influencing pollutant removal is the temperature during biochar production. Processing at lower temperatures (<500 °C) leads to partial carbonization, yielding biochar with smaller pores, diminished surface area, and elevated oxygen functional groups. This type of biochar is proficient at adsorbing inorganic contaminants. Conversely, biochar generated at higher temperatures (>500 °C) generally boasts a larger surface area and may demonstrate improved adsorption capacity for organic pollutants [189, 195]. Common mechanisms by which metal ions adhere to biochar surfaces include cation exchange and complexation with oxygenated functional groups [162, 196].
Choi et al. [159] explored the efficacy of biochar obtained from Spirulina sp. in removing the antibiotic tetracycline from wastewater at various pyrolysis temperatures. They discovered a positive correlation among the surface area, aromaticity, and hydrophobicity of the biochar, with higher pyrolysis temperatures leading to increased levels of these properties. At the highest temperature tested (750 °C), the produced biochar exhibited heightened functional groups, improved metal complexation, and amplified electrostatic and hydrophobic interactions, resulting in a maximum adsorption capacity of 132.8 mg g−1.
In another study conducted by Yang et al. [174], the adsorption characteristics of Pb(II) on biochar derived from the residue of Chlorella sp. (CB) and Spirulina sp. (SP) pyrolysis were investigated. The adsorption process revealed both rapid and slow adsorption phases. Initially, as the pyrolysis temperature increased, there was a decline in adsorption capacity; however, with further temperature increments, the capacity subsequently rose. At a pyrolytic temperature of 600 °C, CB and SP demonstrated maximum adsorption capacities of 131.41 and 154.56 mg g−1, respectively.
6 Economic Analysis of Microalgae Biochar Production and Consumption
The economic analysis of microalgae-derived biochar production involves evaluating the costs, benefits, and overall economic feasibility of converting microalgae biomass into biochar for various applications. A significant portion of the total cost arises from the cultivation of microalgae as feedstocks for biochar production. Therefore, it is crucial to implement strategies to reduce these costs. Some key measures include optimizing cultivation locations, enhancing photosynthetic efficiency, utilizing free sources of carbon dioxide and nutrients, scaling up operations, and developing robust year-round processes. These efforts collectively improve efficiency and reduce technological and material expenses [197]. Additionally, the choice of cultivation systems plays a pivotal role in microalgae production economics. Cultivation is the most cost-intensive stage due to the high energy requirements needed to supply essential growth factors, such as light, nutrients, and stable environmental conditions (e.g., temperature, pH, and low oxygen levels) [198]. Open cultivation systems, for instance, have lower capital and operational costs and yield high-value products but suffer from low productivity due to challenges like suboptimal growth media, nutrient limitations, and environmental fluctuations. Consequently, an economic evaluation of environmental conditions is essential for accurately estimating microalgae productivity and production costs. Banerjee and Ramaswamy [199] demonstrated this through a dynamic process model evaluating the potential productivity of microalgae in the United States. Their findings revealed that key factors, such as areal productivity, nutrient costs, and pond design, significantly influence microalgae production costs, which ranged between $502 and $1074 per ton. Similarly, Banerjee and Ramaswamy [200] conducted a comprehensive economic analysis of microalgae cultivation across various US locations. They assessed start-up and operational costs for commercial-scale production using flat-panel PBRs, revealing costs ranging from $2895 to $9564 per ton. This techno-economic evaluation highlighted that geospatial location, productivity potential, and the engineering design of cultivation systems are critical determinants of production costs. Hossain et al. [201] conducted an economic analysis of a 6-ha coastal area, estimating an annual biomass yield of 220 t. The study showed that annual productivity in PBRs and open ponds was 56 and 28 t ha−1, respectively. The higher productivity of closed systems stems from better control over cultivation parameters. A sensitivity analysis by Acién et al. [202] further explored the impact of microalgae pricing, aiming to identify optimal production values. The study concluded that a selling price of €10 kg−1 would be necessary for producing 200 t of microalgae annually. The harvesting and dewatering of microalgae biomass are critical but cost-intensive processes, accounting for an estimated 20–30 % of total production costs. These costs vary depending on the chosen technology, which is often selected based on the intended end-use of the biomass, such as biofuel production [198]. The high expenses stem from the significant energy requirements, large processing volumes, and small cell size of microalgae, with labor being a major contributor [203]. Automation offers a potential solution to reduce labor costs, though it requires a higher initial investment. Alternatively, cost-effective and energy-efficient flocculation methods are recommended to minimize capital expenditures, whereas energy-intensive centrifugation remains ideal for recovering high-quality, uncontaminated algae [204, 205].
The conversion of microalgae biomass to biochar in a biorefinery often employs pyrolysis, typically operating within a temperature range of 300–700 °C. Pyrolysis is favored for its relatively low cost and straightforward operation [167]. However, evaluating the pyrolysis process requires consideration of several factors, including the characteristics of the microalgae feedstock, facility and machinery costs, labor, energy and maintenance expenses, and the potential revenue from biochar sales [206, 207]. Biochar yield plays a pivotal role in determining the profitability of the pyrolysis process. Slow pyrolysis is particularly recommended for producing high-quality biochar, as its longer residence time maximizes yield and retains carbon properties [167]. To further enhance biochar quality and reduce operational costs, catalytic pyrolysis has emerged as a promising strategy. Pre-treatment of microalgal biomass and the development of advanced reactor designs are additional approaches to improving cost efficiency [208, 209].
Beyond its production, biochar offers significant economic and societal benefits, particularly in regions with limited access to electricity. It supports small- and medium-sized enterprises and frequently provides value that surpasses its monetary cost. Although microalgae-derived biochar exhibits properties comparable to those of terrestrial biochar, comprehensive economic analyses encompassing material and energy flows, production costs, and financial evaluations remain underexplored [205]. According to de Morais et al. [209], the diverse applications of microalgae biochar as a fuel, adsorbent, catalyst, and fertilizer enhance its economic viability. The conversion of biochar to activated carbon, for example, increases its market value to $1188 per ton, contributing 51.11 % of total revenue. Similarly, Marousek et al. [205] conducted a detailed analysis of algae biochar production costs under central European conditions. Their findings revealed that production costs consistently exceeded €110 kg−1, significantly higher than market prices for most common biochars, including high-value hardwood-derived varieties.
7 Challenges and Future Prospective
The growing interest in producing biochar from microalgae feedstocks is driven by their high photosynthetic efficiency, rapid growth, and adaptability to various aquatic environments, including wastewater systems, which can serve a dual purpose of biomass cultivation and wastewater treatment. Furthermore, microalgae-based biochar production holds significant potential for integration into biofuel systems, making it a sustainable pathway for creating versatile biochar products. However, several challenges must be addressed to enhance the viability and efficiency of thermal conversion techniques for microalgae biochar production. One of the primary challenges is the energy-intensive nature of thermal conversion processes, which operate at high temperatures. If not carefully managed, the significant energy demand can render the process economically and environmentally unsustainable. Additionally, precise temperature regulation during thermal conversion is difficult, often leading to process inefficiencies, increased complexity, and additional operational costs. Another major concern is the substantial carbon loss during pyrolysis, one of the most pressing issues in thermal conversion. Studies indicate that thermal degradation and volatilization can result in more than 50 % carbon loss [210], raising questions about the effectiveness of biochar as a carbon sequestration tool. This carbon loss also directly reduces biochar yields, a problem that is particularly pronounced in plant-based biomasses. To address these limitations, emerging technologies such as microwave-assisted pyrolysis have shown promise due to their lower energy consumption, improved energy efficiency, and ability to produce higher biochar yields in a more controllable manner [211, 212]. However, the additional energy demands associated with microwave-assisted processes must be carefully optimized to ensure economic feasibility. Process optimization is another critical strategy for improving the consistency and quality of biochar produced from microalgae. By enhancing char yields and reducing operational costs, optimization techniques can make the thermal conversion process more efficient and economically viable.
The complexity and high cost of microalgae feedstock pose another significant challenge, primarily due to the energy-intensive processes involved in harvesting and drying. Compared to other biomass feedstocks, microalgae require more energy and longer processing times, particularly during the drying stage. This step is critical, as the low concentration of algae in the growth medium, typically between 10 % and 20 %, even after harvesting, necessitates thorough drying before entering the pyrolysis reactor [213]. One potential solution for improving economic viability is the use of wastewater as a growth medium for microalgae. Wastewater not only provides a cost-effective feedstock but also allows microalgae to generate energy-rich components like lipids and carbohydrates, further enhancing biochar production [214]. Recent technological advancements, such as nanotechnology, offer additional opportunities for improving biofuel production and microalgae cultivation. The use of innovative materials like nanoparticles and nanostructures can lead to more efficient thermal conversion processes with higher yields [215]. Similarly, the adoption of novel dewatering and drying technologies such as electrolytic systems, including electroflocculation, electroflotation, and combined electrocoagulation, can help reduce energy consumption and pre-treatment costs [216]. These advancements are particularly crucial for overcoming the energy challenges associated with microalgae processing. It is important to note that the species of microalgae, cultivation techniques, and environmental conditions play a significant role in determining the biomass composition. These factors, in turn, influence the suitability of microalgae-derived biochar for various applications. Additionally, pre-treatment methods can enhance biochar yield and quality, making them valuable for broader applications. Thus, careful consideration of all these parameters is essential for successfully converting microalgal biomass into high-value biochar.
Despite recent advancements, there is still limited information regarding the economic evaluation of thermal conversion processes for microalgae-derived biochar. This research gap makes it difficult to assess the scalability and cost-effectiveness of these technologies, posing a barrier to their industrial adoption and commercialization. A comprehensive economic analysis is essential to address this issue, considering factors, such as feedstock cultivation and harvesting costs, energy consumption, reactor efficiency, utilization of by-products, and market demand for biochar. Bridging these knowledge gaps will be pivotal in advancing microalgae-based biochar production toward large-scale, sustainable applications.
8 Conclusion and Recommendations
The study examined diverse thermochemical techniques for producing biochar from microalgae and explored how production parameters affect the properties and possible uses of the biochar. The results indicated that biochar production from microalgae was notably influenced by feedstock composition and operational parameters, such as heating rate, temperature, and residence time. Lower temperatures resulted in a larger biochar yield but a lower surface area, whereas higher conversion temperatures led to biochar with a higher surface area but reduced biochar yield. Biochar derived from microalgae showed reduced carbon content, surface area, and cation exchange capacities in comparison to biochar obtained from lignocellulosic biomass. Nevertheless, it showcased elevated pH, ash, nitrogen, and extractable inorganic nutrient contents.
Even though there is a lot of interest in producing biofuel from microalgae, researchers have primarily focused on producing bio-oil and refining it. Yet, the fuel that results from this is still a long way from being economically viable. Given the huge potential uses of biochar in agriculture and the environment, biochar production should therefore receive equal attention. Cost analysis studies should be a part of future studies to ascertain the actual cost of making biochar from microalgae.
Acknowledgments
The authors gratefully acknowledge the financial support provided by the National Research Foundation of South Africa and Sasol Ltd (NRF-SASOL).
Conflict of Interest
The authors declare no conflict of interest.
Symbols Used
-
- [°C]
-
- degree Celsius
-
- [h]
-
- hours
-
- [wt %]
-
- Weight %
-
- [m]
-
- meter (s)
-
- [kg]
-
- kilogram
-
- [s]
-
- second
-
- [mg]
-
- milligram
Abbreviations
-
- BET
-
- Brunauer–Emmett–Teller
-
- CEC
-
- cation exchange capacity
-
- DA
-
- deashing microalgae
-
- DTA
-
- differential thermal analysis
-
- FESEM
-
- field emission scanning electron microscopy
-
- FTIR
-
- Fourier transform infrared
-
- HHV
-
- higher heating value
-
- HTC
-
- hydrothermal carbonization
-
- IUPAC
-
- International Union of Pure and Applied Chemistry
-
- LPG
-
- liquefied petroleum gas
-
- MSW
-
- municipal solid waste
-
- PBR
-
- photo-bioreactor
-
- SEM
-
- scanning electron microscopy
-
- TEM
-
- transmission electron microscopy
-
- TGA
-
- thermogravimetry analysis
-
- TRL
-
- technology readiness level
-
- US
-
- United States
-
- WOS
-
- Web of Science
-
- WWTP
-
- wastewater treatment plant
Biography

Sherif Ishola Mustapha is a Postdoctoral Research Fellow at the School of Chemical and Metallurgical Engineering, University of Witwatersrand, South Africa. His expertise spans the production of biofuels, heterogeneous catalysis, process modeling and simulation, wastewater treatment, nanotechnology, and renewable energy. Sherif's current research activities involve the production of biofuels using different thermal conversion processes and the development of supported and unsupported nano-composite catalysts for water and energy application.




