Three Undescribed Protoilludane-Type Sesquiterpene Aryl Esters from Armillaria gallica
Jing-Jing Zhang
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, Yunnan Characteristic Plant Extraction Laboratory, Yunnan Key Laboratory of Research and Development for Natural Products, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Institute of International Rivers and Eco-Security, School of Pharmacy and School of Chemical Science and Technology, Yunnan University, Kunming, 650500 China
Southwest United Graduate School, Kunming, 650092 China
Search for more papers by this authorSong-Xue Yang
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, Yunnan Characteristic Plant Extraction Laboratory, Yunnan Key Laboratory of Research and Development for Natural Products, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Institute of International Rivers and Eco-Security, School of Pharmacy and School of Chemical Science and Technology, Yunnan University, Kunming, 650500 China
Search for more papers by this authorXing-Jie Zhang
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, Yunnan Characteristic Plant Extraction Laboratory, Yunnan Key Laboratory of Research and Development for Natural Products, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Institute of International Rivers and Eco-Security, School of Pharmacy and School of Chemical Science and Technology, Yunnan University, Kunming, 650500 China
Search for more papers by this authorNa Li
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, Yunnan Characteristic Plant Extraction Laboratory, Yunnan Key Laboratory of Research and Development for Natural Products, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Institute of International Rivers and Eco-Security, School of Pharmacy and School of Chemical Science and Technology, Yunnan University, Kunming, 650500 China
Search for more papers by this authorXiao-Ting He
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, Yunnan Characteristic Plant Extraction Laboratory, Yunnan Key Laboratory of Research and Development for Natural Products, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Institute of International Rivers and Eco-Security, School of Pharmacy and School of Chemical Science and Technology, Yunnan University, Kunming, 650500 China
Search for more papers by this authorCorresponding Author
Wei-Lie Xiao
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, Yunnan Characteristic Plant Extraction Laboratory, Yunnan Key Laboratory of Research and Development for Natural Products, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Institute of International Rivers and Eco-Security, School of Pharmacy and School of Chemical Science and Technology, Yunnan University, Kunming, 650500 China
Southwest United Graduate School, Kunming, 650092 China
Search for more papers by this authorCorresponding Author
Li-Jun Cheng
Yunnan Key Laboratory of Gastrodia and Fungi Symbiotic Biology, Zhaotong University, Zhaotong, 65700 China
Search for more papers by this authorCorresponding Author
Xiao-Li Li
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, Yunnan Characteristic Plant Extraction Laboratory, Yunnan Key Laboratory of Research and Development for Natural Products, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Institute of International Rivers and Eco-Security, School of Pharmacy and School of Chemical Science and Technology, Yunnan University, Kunming, 650500 China
Search for more papers by this authorJing-Jing Zhang
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, Yunnan Characteristic Plant Extraction Laboratory, Yunnan Key Laboratory of Research and Development for Natural Products, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Institute of International Rivers and Eco-Security, School of Pharmacy and School of Chemical Science and Technology, Yunnan University, Kunming, 650500 China
Southwest United Graduate School, Kunming, 650092 China
Search for more papers by this authorSong-Xue Yang
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, Yunnan Characteristic Plant Extraction Laboratory, Yunnan Key Laboratory of Research and Development for Natural Products, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Institute of International Rivers and Eco-Security, School of Pharmacy and School of Chemical Science and Technology, Yunnan University, Kunming, 650500 China
Search for more papers by this authorXing-Jie Zhang
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, Yunnan Characteristic Plant Extraction Laboratory, Yunnan Key Laboratory of Research and Development for Natural Products, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Institute of International Rivers and Eco-Security, School of Pharmacy and School of Chemical Science and Technology, Yunnan University, Kunming, 650500 China
Search for more papers by this authorNa Li
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, Yunnan Characteristic Plant Extraction Laboratory, Yunnan Key Laboratory of Research and Development for Natural Products, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Institute of International Rivers and Eco-Security, School of Pharmacy and School of Chemical Science and Technology, Yunnan University, Kunming, 650500 China
Search for more papers by this authorXiao-Ting He
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, Yunnan Characteristic Plant Extraction Laboratory, Yunnan Key Laboratory of Research and Development for Natural Products, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Institute of International Rivers and Eco-Security, School of Pharmacy and School of Chemical Science and Technology, Yunnan University, Kunming, 650500 China
Search for more papers by this authorCorresponding Author
Wei-Lie Xiao
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, Yunnan Characteristic Plant Extraction Laboratory, Yunnan Key Laboratory of Research and Development for Natural Products, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Institute of International Rivers and Eco-Security, School of Pharmacy and School of Chemical Science and Technology, Yunnan University, Kunming, 650500 China
Southwest United Graduate School, Kunming, 650092 China
Search for more papers by this authorCorresponding Author
Li-Jun Cheng
Yunnan Key Laboratory of Gastrodia and Fungi Symbiotic Biology, Zhaotong University, Zhaotong, 65700 China
Search for more papers by this authorCorresponding Author
Xiao-Li Li
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, Yunnan Characteristic Plant Extraction Laboratory, Yunnan Key Laboratory of Research and Development for Natural Products, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Institute of International Rivers and Eco-Security, School of Pharmacy and School of Chemical Science and Technology, Yunnan University, Kunming, 650500 China
Search for more papers by this authorAbstract
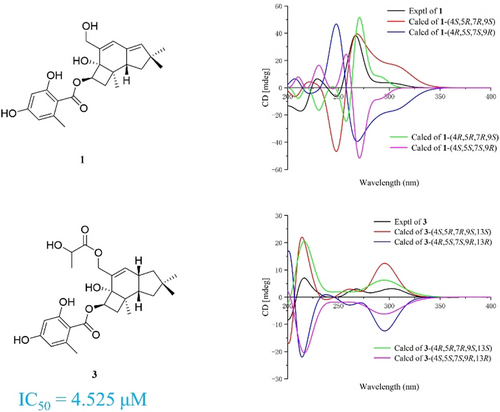
Three previously undescribed protoilludane-type sesquiterpene aryl esters, armillanals A−C (1–3), along with seven known ones (4–10) were obtained from Armillaria gallica Marxm. & Romagn. Compounds 1 and 2 were a rare class of sesquiterpenes featuring the Δ2(3) and Δ12(13)-protoilludane skeleton. Their structures were established by extensive spectroscopic methods. Based on electronic circular dichroism (ECD) calculations, the absolute configurations of three new compounds (1–3) were determined. The anti-inflammatory activity of compounds 1–10 was screened and compound 3 could dose-dependently decrease the level of lactate dehydrogenase, showing IC50 value of 4.525 μM.
Graphical Abstract
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv202401303-sup-0001-misc_information.pdf3.2 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. R. Carroll, B. R. Copp, R. A. Davis, R. A. Keyzers, M. R. Prinsep, Nat. Prod. Rep. 2020, 37, 175–223.
- 2L. Liu, L. Wang, L. Bao, J. Ren, B. B. Basnet, R. Liu, L. He, J. Han, W. B. Yin, H. Liu, Org. Lett. 2017, 19, 1494–1495.
- 3C. C. Chen, Y. H. Kuo, J. J. Cheng, P. J. Sung, C. L. Ni, C. C. Chen, C. C. Shen, Molecules 2015, 20, 9994–10003.
- 4S. Z. Lou, J. Feng, R. Yang, Y. P. Li, L. Gao, G. Du, H. Y. Yang, Q. F. Hu, W. B. Zhou, L. S. Wang, W. G. Wang, J. Asian Nat. Prod. Res. 2022, 24, 59–65.
- 5X. Yin, T. Feng, J. K. Liu, Nat. Prod. Bioprospect. 2012, 2, 245–248.
10.1007/s13659-012-0077-1 Google Scholar
- 6M. Bohnert, S. Miethbauer, H. M. Dahse, J. Ziemen, M. Nett, D. Hoffmeister, Bioorg. Med. Chem. Lett. 2011, 21, 2003–2006.
- 7M. Alves, I. F. R. Ferreira, J. Dias, V. Teixeira, A. Martins, M. Pintado, Planta Med. 2012, 78, 1707–1718.
- 8C. Z. Song, X. Yan, J. Anhui Agric. Sci. 2010, 38, 5119–5120.
- 9L. W. Gao, J. W. Wang, J. Food Biochem. 2011, 36, 139–148.
- 10I. Momose, R. Sekizawa, N. Hosokawa, H. Iinuma, S. Maisui, H. Nakamura, H. Naganawa, M. Hamada, T. Takeuchi, J. Antibiot. 2000, 53, 137–143.
- 11H. T. Li, Y. Sun, M. Wang, J. Y. Chen, H. Zhou, Z. T. Ding, Chin. J. Org. Chem. 2021, 41, 4493–4497.
10.6023/cjoc202107006 Google Scholar
- 12J. S. Yang, Y. L. Su, Y. L. Wang, X. Z. Feng, X. T. Liang, Planta Med. 1991, 57, 478–480.
- 13J. S. Yang, Y. L. Su, Y. L. Wang, X. Z. Feng, D. Q. Yu, X. T. Liang, J. Pharm. Sci. 1991, 26, 117–122.
- 14W. J. Guo, S. X. Guo, J. S. Yang, X. M. Chen, P. G. Xiao, Biochem. Syst. Ecol. 2007, 35, 790–793.
- 15J. A. Vaz, L. Barros, A. Martins, C. Santos-Buelga, M. H. Vasconcelos, I. C. F. R. Ferreira, Food Chem. 2011, 126, 610–616.
- 16J. Sonnenbichler, J. J. Guillaumin, H. Peipp, D. Schwarz, Eur. J. For. Pathol. 2007, 27, 241–249.
10.1111/j.1439-0329.1997.tb00866.x Google Scholar
- 17D. M. X. Donnelly, T. Konishi, O. Dunne, P. Cremin, Phytochemistry 1997, 44, 1473–1478.
- 18D. M. X. Donnelly, R. M. Hutchinson, Phytochemistry 1990, 29, 179–182.
- 19S. L. Midland, R. R. Izac, R. M. Wing, A. I. Zaki, D. E. Munnecke, J. J. Sims, Tetrahedron Lett. 1982, 23, 2515–2518.
- 20M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, D. J. Fox, Gaussian 09, Revision E.01, Gaussian, Inc., Wallingford CT 2013.
- 21T. Bruhn, G. Pescitelli, Chirality 2016, 28, 749–749.
- 22C. L. Nord, A. Menkis, C. Lendel, R. Vasaitis, A. Broberg, Phytochemistry 2014, 102, 197–204.