α-Glucosidase Inhibitory Activity of Prenylated Pyranocoumarins from Clausena excavata: Mechanism of Action, ADMET and Molecular Docking
Abstract
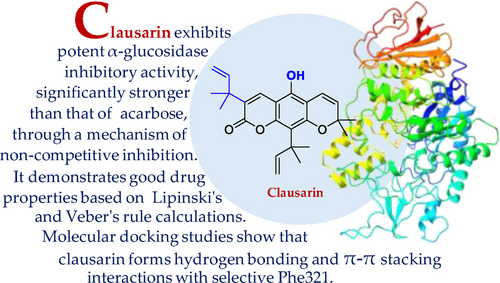
Three naturally occurring prenylated pyranocoumarins, nordentatin (1), dentatin (2), and clausarin (3), isolated from the roots of Clausena excavata (Family Rutaceae), and O-methylclausarin (4) which was obtained by methylation of 3, were investigated for their α-glucosidase inhibitory activity. The mechanism of action and the in silico prediction of their physicochemical and ADMET properties as well as the molecular docking were also studied. Compounds 1–4 exhibited stronger α-glucosidase inhibitory activity than the positive control, acarbose, through a non-competitive mechanism. Among them, 3 exhibited the highest activity, with an IC50 of 8.36 μM, which is significantly stronger than that of acarbose (IC50=430.35 μM). The prenyl group on C-3 and the hydroxyl group on C-5 in 3 may play important roles in enhancing the activity. Calculated physicochemical and ADMET parameters of 1–4 satisfied the Lipinski's and Veber's rules. Molecular simulation analysis indicated they are promising drug candidates with no hepatotoxicity. Compound 3 exhibited potent activity in the experiment and demonstrated good drug properties based on the calculations. A molecular docking study revealed that 3 showed H-bonding and π-π stacking interactions with selective Phe321, as well as interactions with thirteen other amino acid residues of the α-glucosidase.
Graphical Abstract
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.