Pyrones Isolated from Annona Acutiflora Exhibit Promising Cytotoxic Effects on Cancer Cell Lines
MSc. Diogo Folly
Laboratório de Tecnologia de Produtos Naturais, LTPN, Departamento de Tecnologia Farmacêutica, Faculdade de Farmácia, Universidade Federal Fluminense, Rua, Mario Viana, 523, CEP: 24241-000 Santa Rosa, Niterói, RJ, Brazil
Universidade Federal Fluminense, Faculdade de Farmácia, Departamento de Tecnologia Farmacêutica e Cosméticos, CEP: 24241-000 Niterói-RJ, Brazil
Laboratório de Química de Produtos Naturais, Universidade Federal Fluminense, Rua São João Batista, 2–188, CEP: 24020-141 Niterói, RJ, Brazil
Programa de Pós-Graduação em Biotecnologia Vegetal e Bioprocessos, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brazil
These authors contributed equally to this work.
Contribution: Data curation (equal), Formal analysis (equal), Investigation (lead), Methodology (equal), Project administration (lead), Writing - original draft (lead), Writing - review & editing (equal)
Search for more papers by this authorSamille Candido da Silva
Department of Basic Science, Campus Universitário de Nova Friburgo, Federal Fluminense, University Nova, Friburgo, CEP 28625-650 Brazil
Universidade Federal Fluminense, Department of Basic Sciences, Nova Friburgo Health Institute, CEP 28625-650 Nova Friburgo-RJ, Brazil
These authors contributed equally to this work.
Search for more papers by this authorGabriela Dinis
Laboratório de Química de Produtos Naturais, Universidade Federal Fluminense, Rua São João Batista, 2–188, CEP: 24020-141 Niterói, RJ, Brazil
Contribution: Investigation (supporting), Methodology (supporting), Writing - original draft (supporting), Writing - review & editing (supporting)
Search for more papers by this authorGabriel Ouverney
Postgraduate Program in Sciences Applied to Health Products, Faculty of Pharmacy, Federal Fluminense University, Niterói, CEP 24020-141 RJ, Brazil
Search for more papers by this authorGuilherme Freimann Wermelinger
Department of Basic Science, Campus Universitário de Nova Friburgo, Federal Fluminense, University Nova, Friburgo, CEP 28625-650 Brazil
Universidade Federal Fluminense, Department of Basic Sciences, Nova Friburgo Health Institute, CEP 28625-650 Nova Friburgo-RJ, Brazil
Search for more papers by this authorPhD. Lucas Silva Abreu
Laboratório de Química de Produtos Naturais, Universidade Federal Fluminense, Rua São João Batista, 2–188, CEP: 24020-141 Niterói, RJ, Brazil
Contribution: Formal analysis (lead), Supervision (equal), Writing - review & editing (equal)
Search for more papers by this authorCorresponding Author
PhD. Bruno Kaufmann Robbs
Department of Basic Science, Campus Universitário de Nova Friburgo, Federal Fluminense, University Nova, Friburgo, CEP 28625-650 Brazil
Universidade Federal Fluminense, Department of Basic Sciences, Nova Friburgo Health Institute, CEP 28625-650 Nova Friburgo-RJ, Brazil
Contribution: Data curation (equal), Formal analysis (equal), Funding acquisition (equal), Supervision (equal), Writing - review & editing (equal)
Search for more papers by this authorCorresponding Author
PhD. Leandro Rocha
Laboratório de Tecnologia de Produtos Naturais, LTPN, Departamento de Tecnologia Farmacêutica, Faculdade de Farmácia, Universidade Federal Fluminense, Rua, Mario Viana, 523, CEP: 24241-000 Santa Rosa, Niterói, RJ, Brazil
Universidade Federal Fluminense, Faculdade de Farmácia, Departamento de Tecnologia Farmacêutica e Cosméticos, CEP: 24241-000 Niterói-RJ, Brazil
Programa de Pós-Graduação em Biotecnologia Vegetal e Bioprocessos, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brazil
Contribution: Data curation (equal), Formal analysis (equal), Funding acquisition (lead), Project administration (lead), Supervision (equal), Writing - review & editing (lead)
Search for more papers by this authorMSc. Diogo Folly
Laboratório de Tecnologia de Produtos Naturais, LTPN, Departamento de Tecnologia Farmacêutica, Faculdade de Farmácia, Universidade Federal Fluminense, Rua, Mario Viana, 523, CEP: 24241-000 Santa Rosa, Niterói, RJ, Brazil
Universidade Federal Fluminense, Faculdade de Farmácia, Departamento de Tecnologia Farmacêutica e Cosméticos, CEP: 24241-000 Niterói-RJ, Brazil
Laboratório de Química de Produtos Naturais, Universidade Federal Fluminense, Rua São João Batista, 2–188, CEP: 24020-141 Niterói, RJ, Brazil
Programa de Pós-Graduação em Biotecnologia Vegetal e Bioprocessos, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brazil
These authors contributed equally to this work.
Contribution: Data curation (equal), Formal analysis (equal), Investigation (lead), Methodology (equal), Project administration (lead), Writing - original draft (lead), Writing - review & editing (equal)
Search for more papers by this authorSamille Candido da Silva
Department of Basic Science, Campus Universitário de Nova Friburgo, Federal Fluminense, University Nova, Friburgo, CEP 28625-650 Brazil
Universidade Federal Fluminense, Department of Basic Sciences, Nova Friburgo Health Institute, CEP 28625-650 Nova Friburgo-RJ, Brazil
These authors contributed equally to this work.
Search for more papers by this authorGabriela Dinis
Laboratório de Química de Produtos Naturais, Universidade Federal Fluminense, Rua São João Batista, 2–188, CEP: 24020-141 Niterói, RJ, Brazil
Contribution: Investigation (supporting), Methodology (supporting), Writing - original draft (supporting), Writing - review & editing (supporting)
Search for more papers by this authorGabriel Ouverney
Postgraduate Program in Sciences Applied to Health Products, Faculty of Pharmacy, Federal Fluminense University, Niterói, CEP 24020-141 RJ, Brazil
Search for more papers by this authorGuilherme Freimann Wermelinger
Department of Basic Science, Campus Universitário de Nova Friburgo, Federal Fluminense, University Nova, Friburgo, CEP 28625-650 Brazil
Universidade Federal Fluminense, Department of Basic Sciences, Nova Friburgo Health Institute, CEP 28625-650 Nova Friburgo-RJ, Brazil
Search for more papers by this authorPhD. Lucas Silva Abreu
Laboratório de Química de Produtos Naturais, Universidade Federal Fluminense, Rua São João Batista, 2–188, CEP: 24020-141 Niterói, RJ, Brazil
Contribution: Formal analysis (lead), Supervision (equal), Writing - review & editing (equal)
Search for more papers by this authorCorresponding Author
PhD. Bruno Kaufmann Robbs
Department of Basic Science, Campus Universitário de Nova Friburgo, Federal Fluminense, University Nova, Friburgo, CEP 28625-650 Brazil
Universidade Federal Fluminense, Department of Basic Sciences, Nova Friburgo Health Institute, CEP 28625-650 Nova Friburgo-RJ, Brazil
Contribution: Data curation (equal), Formal analysis (equal), Funding acquisition (equal), Supervision (equal), Writing - review & editing (equal)
Search for more papers by this authorCorresponding Author
PhD. Leandro Rocha
Laboratório de Tecnologia de Produtos Naturais, LTPN, Departamento de Tecnologia Farmacêutica, Faculdade de Farmácia, Universidade Federal Fluminense, Rua, Mario Viana, 523, CEP: 24241-000 Santa Rosa, Niterói, RJ, Brazil
Universidade Federal Fluminense, Faculdade de Farmácia, Departamento de Tecnologia Farmacêutica e Cosméticos, CEP: 24241-000 Niterói-RJ, Brazil
Programa de Pós-Graduação em Biotecnologia Vegetal e Bioprocessos, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brazil
Contribution: Data curation (equal), Formal analysis (equal), Funding acquisition (lead), Project administration (lead), Supervision (equal), Writing - review & editing (lead)
Search for more papers by this authorAbstract
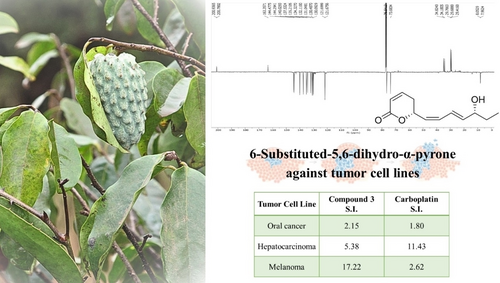
This work discusses the ongoing challenge of cancer, focusing on therapy issues such as chemotherapy resistance and adverse drug effects. It emphasizes the need for new anticancer agents with improved efficacy and fewer side effects, exploring natural products from plant sources. The Annonaceae family, specifically the Annona genus, is highlighted for its medicinal properties, including anti-inflammatory and anticancer effects. The study focuses on the isolation and elucidation of the substances present in Annona acutiflora leaves. The methodology involves chromatographic and spectroscopy techniques. The isolated compounds, (6S)-5′-oxohepten-1′E,3′E-dienyl)-5,6-dihydro-2H-pyran-2-one (1), (6R)-5′-oxohepten-1′Z,3′E-dienyl)-5,6-dihydro-2H-pyran-2-one (2) and (6R)-5′-oxohepten-1′Z,3′E-dienyl)-5,6-dihydro-2H-pyran-2-one (3) were investigated for cytotoxicity assays on cancer cell lines and normal cells. Results show promising cytotoxic activity, particularly with compound 3, demonstrating potential activity against oral cancer (43.18 μM), hepatocarcinoma (17.24 μM), melanoma (5.39 μM), and colon cancer (59.03 μM). The compound outperforms carboplatin in selectivity against oral cancer (S. I. 2.15) and melanoma (S. I. 17.22). The study concludes by suggesting the potential of these α-pyrones as effective and less toxic alternatives for cancer therapy.
Graphical Abstract
Conflict of Interests
The authors declare that they have no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv202400572-sup-0001-misc_information.pdf2.1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. S. Hecht, Nat. Rev. Cancer 2003, 3 (10), 733–44. https://doi.org/10.1038/nrc1190.
- 2Q. Xu, Q. Dong, C. He, W. Liu, L. Sun, J. Liu, C. Xing, X. Li, B. Wang, Y. Yuan, PLoS One 2014, 9 (4), e95249.
- 3P. Economopoulou, G. Dimitriadis, A. Psyrri, Cancer Treat. Rev. 2015, 41 (1), 1–8.
- 4Z. Pénzváltó, A. Lánczky, J. Lénárt, N. Meggyesházi, T. Krenács, N. Szoboszlai, C. Denkert, I. Pete, B. Győrffy, BMC Cancer 2014, 14 (1), 1–0.
- 5S. Zhang, X. Liu, T. Bawa-Khalfe, L. S. Lu, Y. L. Lyu, L. F. Liu, E. T. Yeh, Nat. Med. 2012, 18 (11), 1639–42.
- 6R. A. Elrayess, H. N. El-Hak, Cancer Studies and Molecular Medicine. 2019, 5 (1), 14–25.
- 7A. A. Dev, S. M. Joseph, J. Indian Chem. Soc. 2021, 98 (12), 100231.
10.1016/j.jics.2021.100231 Google Scholar
- 8K. Pumiputavon, T. Chaowasku, C. Saenjum, M. Osathanunkul, B. Wungsintaweekul, K. Chawansuntati, P. Lithanatudom, J. Wipasa, In Vitro Cellular and Developmental Biology-Animal 2019, 55, 723–32.
- 9D. O. Leite, C. de F. A. Nonato, C. J. Camilo, N. K. de Carvalho, M. G. da Nobrega, R. C. Pereira, J. G. da Costa, Curr. Pharm. Des. 2020, 26 (33), 4056–91.
- 10N. N. das Chagas Lima, C. C. Faustino, K. J. Allahdadi, L. S. de Aragão França, L. C. Pinto, PharmaNutrition. 2022, 20, 100295.
10.1016/j.phanu.2022.100295 Google Scholar
- 11D. Folly, F. P. Machado, R. Esteves, J. L. Duarte, R. A. Cruz, A. E. Oliveira, R. M. Ferreira, R. N. Souto, M. G. Santos, J. C. Carvalho, B. M. Ruppelt, J. Essent. Oil Res. 2021, 33 (6), 559–66.
- 12D. Carmona, J. Sáez, H. Granados, E. Pérez, S. Blair, A. Angulo, B. Figadere, Nat. Prod. Res. 2003, 17 (4), 275–80.
- 13M. Matsuda, Y. Endo, S. Fushiya, T. Endo, S. Nozoe, Chorisia crispiflora’. Heterocycles (Sendai). 1994, 38 (6), 1229–32.
- 14S. A. Gharat, M. Momin, C. Bhavsar, Crit. Rev. Ther. Drug Carrier Syst. 2016, 33 (4), 363–400. doi: 10.1615/CritRevTherDrugCarrierSyst.2016016272.PMID:27910740.
- 15A. Gupta, F. Gomes, P. Lorigan, Melanoma Management. 2017, 4 (2), 125–36. https://doi.org/10.2217/mmt-2017-0003.
- 16K. Ikeda, Hepatology Research. 2019, 49 (1), 14–32. https://doi.org/10.1111/hepr.13259.
- 17I. Ali, A. W. Wani, K. Saleem, A. Haque, Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents). 2013, 13 (2), 296–306. https://doi.org/10.2174/187152013804711245.
- 18A. C. Fonseca, L. N. de Queiroz, J. Sales Felisberto, Y. Jesse Ramos, A. Mesquita Marques, G. F. Wermelinger, B. Pontes, D. de Lima Moreira, B. K. Robbs, Nat. Prod. Res. 2021, 35 (24), 6163–7. https://doi.org/10.1080/14786419.2020.1831494.
- 19T. Q. Machado, J. R. S. Felisberto, E. F. Guimarães, G. A. Queiroz, A. C. C. D. Fonseca, Y. J. Ramos, A. M. Marques, D. L. Moreira, B. K. Robbs, Nat. Prod. Res. 2022, 36 (6), 1636–1640. doi: 10.1080/14786419.2021.1895148.
- 20L. N. de Queiroz, A. C. C. Da Fonseca, G. F. Wermelinger, D. P. D. da Silva, A. C. R. R. Pascoal, A. C. H. F. Sawaya, E. C. P. de Almeida, B. S. do Amaral, D. de Lima Moreira, B. K. Robbs, J. Ethnopharmacol. 2023, 303, 116043. doi: 10.1016/j.jep.2022.116043.
- 21T. Q. Machado, M. E. D. Lima, R. C. da Silva, A. L. Macedo, L. N. de Queiroz, B. R. P. Angrisani, A. C. C. da Fonseca, P. R. Câmara, V. V.-H. Rabelo, C. A. Carollo, et al., Biomedicine 2023, 11, 1914. https://doi.org/10.3390/biomedicines11071914.
- 22R. I. Geran, N. H. Greenberg, M. M. Macdonald, A. M. Schumacher, Cancer Chemother. Rep. 1972, 3 (III), 1–103.
- 23J. P. Machiels, C. R. Leemans, W. Golusinski, C. Grau, L. Licitra, V. Gregoire, Ann. Oncol. 2020, 31 (11), 1462–75.
- 24A. L. Macedo, D. P. da Silva, D. L. Moreira, L. N. de Queiroz, T. R. Vasconcelos, G. F. Araujo, M. A. Kaplan, S. S. Pereira, E. C. de Almeida, A. L. Valverde, B. K. Robbs, Biomed. Pharmacother. 2019, 110, 342–52.
- 25B. C. Zorzanelli, G. Ouverney, F. P. Pauli, A. C. da Fonseca, E. C. de Almeida, D. G. de Carvalho, P. A. Possik, V. W. Rabelo, P. A. Abreu, B. Pontes, V. F. Ferreira, Molecules. 2022, 27 (16), 5148.
- 26P. A. Segun, O. O. Ogbole, F. M. Ismail, L. Nahar, A. R. Evans, E. O. Ajaiyeoba, S. D. Sarker, Phytother. Res. 2019, 33 (1), 159–66.
- 27F. Sangermano, M. Masi, M. Vivo, P. Ravindra, A. Cimmino, A. Pollice, A. Evidente, V. Calabrò, Toxicol. in Vitro 2019, 61, 104614.
- 28L. D. Juliawaty, P. N. Ra'idah, S. Abdurrahman, E. Hermawati, A. Alni, M. I. Tan, H. Ishikawa, Y. M. Syah, J. Nat. Med. 2020, 74, 584–90.
- 29F. Torrens, G. Castellano, Curr. Drug Discovery Technol. 2020, 17 (2), 166–82.