Two New Eremophilane-Type Sesquiterpenoids from Ligularia sagitta
Abstract
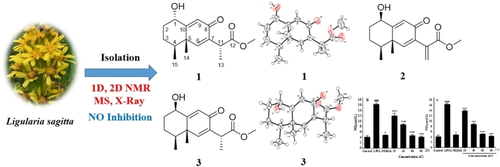
Two new eremophilane-type sesquiterpenoids, sagittacins F and G (1 and 2), together with one known isomer of sagittacin F (3) were isolated from the leaves and stems of Ligularia sagitta. Their structures were elucidated by interpretation of spectroscopic data and the absolute configurations of 1 and 3 were determined by X-ray spectroscopy. Compound 1 belongs to a rare class of eremophilane-type sesquiterpenoid featuring an α-oriented hydroxy group at C-1. A nitric oxide (NO) production inhibitory assay was applied to evaluate their anti-inflammatory activities by using LPS-induced RAW 264.7 cells. Compounds 2 and 3 exhibited modest NO production inhibitions with IC50 values of 45.15±2.72 and 49.83±2.34 μM, respectively.
Graphical Abstract
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.