Synthetic and Medicinal Perspective of 1,2,4-Triazole as Anticancer Agents
Bhagyashri Rathod
School of Pharmacy and Technology Management, SVKM's NMIMS, Hyderabad, Telangana, 509301 India
Search for more papers by this authorCorresponding Author
Kapil Kumar
School of Pharmacy and Technology Management, SVKM's NMIMS, Hyderabad, Telangana, 509301 India
School of Pharmaceutical Sciences, Apeejay Stya University, Gurugram, Haryana, 122103 India
Search for more papers by this authorBhagyashri Rathod
School of Pharmacy and Technology Management, SVKM's NMIMS, Hyderabad, Telangana, 509301 India
Search for more papers by this authorCorresponding Author
Kapil Kumar
School of Pharmacy and Technology Management, SVKM's NMIMS, Hyderabad, Telangana, 509301 India
School of Pharmaceutical Sciences, Apeejay Stya University, Gurugram, Haryana, 122103 India
Search for more papers by this authorAbstract
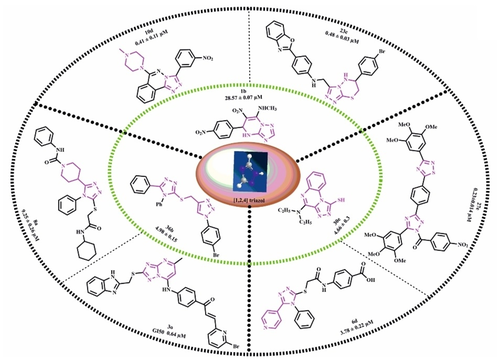
Cancer is still one of the leading causes of death worldwide. Many researchers are working to design and develop heterocyclic-based drugs with the potential to treat cancer at various stages. There is always an unmet need to discover new anticancer drugs to treat different types of cancer. Based on literature reports, it was evident that hybrid 1,2,4-triazoles exhibit a wide spectrum of biological potential (particularly potent anticancer activity), as compared to the corresponding monocyclic compounds. In this review, we summarized fused/hybrid 1,2,4-triazole and poly-functionalized 1,2,4-triazoles as anticancer agents. Triazole may be fused with other heterocyclic scaffolds like pyrimidine, pyridine, piperidine, quinoxaline, quinazoline, thiadiazine, indole, phthalazine etc. A clear explanation of the method of synthesis, structure-activity relationship, and in vitro results plus the inhibitory concentration of new molecules are focused on in this review article.
Graphical Abstract
Conflict of interest
Authors declare no conflict of interest for this article.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1B. Karami, M. Farahi, Z. Banaki, ‘A New Protocol for Catalyst-Free Regioselective Synthesis Of 5, 9-Dihydropyrimido [5, 4-E] [1, 2, 4] Triazolo [1, 5-A] Pyrimidine-6, 8 (4H, 7H)-Diones’, Synlett. 2015, 26, 741–744.
- 2F. H. Al-Ostoot, M. Al-Ghorbani, S. A. Khanum, ‘Recent Investigation on Heterocycles with One Nitrogen [Piperidine, Pyridine and Quinoline], Two Nitrogen [1, 3, 4-Thiadiazole and Pyrazole] and Three Nitrogen [1, 2, 4-Triazole]: A Review’, J. Iran. Chem. Soc. 2022, 19, 23–54.
- 3R. Aggarwal, G. Sumran, ‘An Insight on Medicinal Attributes Of 1, 2, 4-Triazoles’, Eur. J. Med. Chem. 2020, 205, 112652.
- 4R. Sathyanarayana, B. Poojary, ‘Exploring Recent Developments On 1, 2, 4-Triazole: Synthesis and Biological Applications’, J. Chin. Chem. Soc. 2020, 67, 459–477.
- 5M. V. Slivka, N. I. Korol, M. M. Fizer, ‘Fused Bicyclic 1, 2, 4-Triazoles with One Extra Sulfur Atom: Synthesis, Properties, and Biological Activity’, J. Heterocycl. Chem. 2020, 57, 3236–3254.
- 6J. Li, J. Zhang, ‘The Antibacterial Activity Of 1, 2, 3-Triazole-And 1, 2, 4-Triazole-Containing Hybrids Against Staphylococcus Aureus: An Updated Review (2020-Present)’, Curr. Top. Med. Chem. 2022, 22, 41–63.
- 7M. Strzelecka, P. Swiątek, ‘1, 2, 4-Triazoles as Important Antibacterial Agents’, Pharmaceuticals 2021, 14, 224.
- 8X. Ge, Z. Xu, ‘1, 2, 4-Triazole Hybrids with Potential Antibacterial Activity Against Methicillin-Resistant Staphylococcus Aureus’ Arch. Pharm. 2021, 354, 2000223.
- 9A. A. Othman, M. Kihel, S. Amara, ‘1, 3, 4-Oxadiazole, 1, 3, 4-Thiadiazole and 1, 2, 4-Triazole Derivatives as Potential Antibacterial Agents’, Arab. J. Chem. 2019, 12, 1660–1675.
- 10R. R. França, C. A. C. Menozzi, F. S. Castelo-Branco, L. V. B. Hoelz, N. Boechat, ‘The Medicinal Chemistry Of 3-Nitro-1, 2, 4-Triazoles: Focus on Infectious Diseases’, Curr. Top. Med. Chem. 2021, 21, 2072–2100.
- 11S. A. El-Sebaey, ‘Recent Advances In 1, 2, 4-Triazole Scaffolds as Antiviral Agents’, ChemistrySelect 2020, 5, 11654–11680.
- 12A. Pachuta-Stec, ‘Antioxidant Activity Of 1, 2, 4-Triazole and Its Derivatives: A Mini-Review’, Mini-Rev. Med. Chem. 2022, 22, 1081–1094.
- 13T. Huang, H. Jiang, Y. Zhao, J. He, H. Cheng, C. J. Martyniuk, ‘A Comprehensive Review Of 1, 2, 4-Triazole Fungicide Toxicity in Zebrafish (Danio Rerio): A Mitochondrial and Metabolic Perspective’, Sci. Total Environ. 2021, 809, 151177.
- 14Z. Kazeminejad, M. Marzi, A. Shiroudi, S. A. Kouhpayeh, M. Farjam, E. Zarenezhad, ‘Novel 1, 2, 4-Triazoles as Antifungal Agents’, BioMed Res. Int. 2022, 39.
- 15C. Tratrat, ‘1, 2, 4-Triazole: A Privileged Scaffold for The Development of Potent Antifungal Agents-A Brief Review’, Curr. Top. Med. Chem. 2020, 20, 2235–2258.
- 16Y. Cao, H. Lu, ‘Advances in The Application Of 1, 2, 4-Triazole-Containing Hybrids as Anti-Tuberculosis Agents’, Future Med. Chem. 2021, 13, 2107–2124.
- 17Y. Wang, D. Liu, ‘An Important Potential Anti-Epileptic/Anticonvulsant Active Group: A Review Of 1, 2, 4-Triazole Groups and Their Action’, Drug Res. 2022, 72, 131–138.
- 18Y. L. Fan, X. Ke, M. Liu, ‘Coumarin-Triazole Hybrids and Their Biological Activities’, J. Heterocycl. Chem. 2018, 55, 791–802.
- 19X. Wen, Y. Zhou, J. Zeng, X. Liu, ‘Recent Development Of 1, 2, 4-Triazole-Containing Compounds as Anticancer Agents’, Curr. Top. Med. Chem. 2020, 20, 1441–1460.
- 20R. Kaur, A. Ranjan Dwivedi, B. Kumar, V. Kumar, ‘Recent Developments On 1, 2, 4-Triazole Nucleus In Anticancer Compounds: A Review’, Anti-Cancer Agents Med. Chem. 2016, 16, 465–489.
- 21S. Nasri, M. Bayat, K. Kochia, ‘Strategies for Synthesis Of 1, 2, 4-Triazole-Containing Scaffolds Using 3-Amino-1, 2, 4-Triazole’, Mol. Diversity 2022, 26, 717–739.
- 22W. Zafar, S. H. Sumrra, Z. H. Chohan, ‘A Review: Pharmacological Aspects of Metal Based 1, 2, 4-Triazole Derived Schiff Bases’, Eur. J. Med. Chem. 2021, 222, 113602.
- 23N. Korol, M. Slivka, O. Holovko-Kamoshenkova, ‘Recent Advances In 1, 2, 4-Triazole Ring Construction Via Cycloaddition Reactions’, Heterocycles 2021, 102, 2099–2117.
- 24Q. Zhang, C. He, S. Pang, ‘Synthesis of Heterocyclic (Triazole, Furoxan, Furazan) Fused Pyridazine Di−N-Oxides Via Hypervalent Iodine Oxidation’, New J. Chem. 2022, 46, 14324–14327.
- 25S. M. Riyadh, S. M. Gomha, ‘Two Decades of The Synthesis of Mono-And Bis-Aminomercapto [1, 2, 4] Triazoles’, RSC Adv. 2020, 10, 24994–25012.
- 26A. Abdelli, S. Azzouni, R. Plais, A. Gaucher, M. L. Efrit, D. Prim, ‘Recent Advances in The Chemistry Of 1, 2, 4-Triazoles: Synthesis, Reactivity and Biological Activities’, Tetrahedron Lett. 2021, 86, 153518.
- 27J. R. Cox, S. Woodcock, I. H. Hillier, M. A. Vincent, ‘Tautomerism Of 1,2,3- and 1,2,4-Triazole in The Gas Phase and In Aqueous Solution: A Combined Ab Initio Quantum Mechanics and Free Energy Perturbation Study’, J. Phys. Chem. 1990, 94, 5499–5501.
- 28A. A. Boezio, K. Andrews, C. Boezio, M. Chu-Moyer, K. W. Copeland, E. F. DiMauro, R. S. Foti, R. T. Fremeau Jr, H. Gao, S. Geuns-Meyer, R. F. Graceffa, H. Gunaydin, H. Huang, D. S. La, J. Ligutti, B. D. Moyer, E. A. Peterson, V. Yu, M. M. Weiss, ‘1,2,4-Triazolsulfone: A Novel Isosteric Replacement of Acylsulfonamides in The Context of Na(V) Inhibition’, Bioorg. Med. Chem. Lett. 2018, 28, 2103–2108.
- 29S. Hanafi, D. Trache, S. Abdous, Z. Bensalem, A. Mezroua, ‘5-Nitro-1, 2, 4-Triazole-3-One: A Review of Recent Advances’, Chin. J. Energet. Mater. 2019, 27, 326–347.
- 30G. H. Zhang, J. M. Yuan, G. Qian, C. X. Gu, K. Wei, D. L. Mo, J. K. Qin, Y. Peng, Z. P. Zhou, C. X. Pan, G. F. Su, ‘Phthalazino [1, 2-B] Quinazolinones As P53 Activators: Cell Cycle Arrest, Apoptotic Response and Bak-Bcl-Xl Complex Reorganization In Bladder Cancer Cells’, J. Med. Chem. 2017, 60, 6853–6866.
- 31N. H. Nam, K. Parang, ‘Current Targets for Anticancer Drug Discovery’, Curr. Drug Targets 2003, 4, 159–179.
- 32S. Martinez-Gonzalez, S. Rodriguez-Aristegui, C. A. G. de la Oliva, A. I. Hernandez, E. G. Cantalapiedra, C. Varela, A. B. Garcia, O. Rabal, J. Oyarzabal, J. R. Bischoff, J. Klett, M. I. Albarran, A. Cebria, N. Ajenjo, B. Garcia-Serelde, E. Gomez-Casero, M. Cuadrado-Urbano, D. Cebrian, C. Blanco-Aparicio, J. Pastor, ‘Discovery of Novel Triazolo [4,3-B] Pyridazin-3-Yl-Quinoline Derivatives as PIM Inhibitors’, Eur. J. Med. Chem. 2019, 168, 87–109.
- 33I. H. Eissa, A. M. Metwaly, A. Belal, A. B. M. Mehany, R. R. Ayyad, K. El-Adl, H. A. Mahdy, M. S. Taghour, K. M. A. El-Gamal, M. E. El-Sawah, S. A. Elmetwally, M. A. Elhendawy, M. M. Radwan, M. A. El-Sohly, ‘Discovery and Antiproliferative Evaluation of New Quinoxalines as Potential DNA Intercalators and Topoisomerase II Inhibitors’, Arch. Pharm. Chem. Life Sci. 2019, 352, 1900123.
- 34A. M. Mohamed, W. A. El-Sayed, A. A. Ibrahim, N. A. Abdel-Hafez, K. A. Ali, S. F. Mohamed, ‘Recent Trends in The Chemistry Of [1, 2, 4] Triazole [1, 5-A] Pyrimidines’, Org. Prep. Proced. Int. 2021, 53, 211–239.
- 35F. Safari, M. Bayat, S. Nasri, S. Karami, ‘Synthesis and Evaluation of Anti-Tumor Activity of Novel Triazolo [1, 5-A] Pyrimidine on Cancer Cells by Induction of Cellular Apoptosis and Inhibition of Epithelial-To-Mesenchymal Transition Process’, Bioorg. Med. Chem. Lett. 2020, 30, 127111.
- 36S. Wang, X. B. Ma, X. H. Yuan, B. Yu, Y. C. Xu, H. M. Liu, ‘Discovery of New [1, 2, 4] Triazolo [1,5-A] Pyrimidine Derivatives That Kill Gastric Cancer Cells Via Mitochondrial Pathway’, Eur. J. Med. Chem. 2020, 203, 112630.
- 37I. Grieco, M. Bissaro, D. B. Tiz, D. I. Perez, C. Perez, A. Martinez, S. Redenti, E. Mariotto, R. Bortolozzi, G. Viola, G. Cozza, ‘Developing Novel Classes of Protein Kinase CK1d Inhibitors by Fusing [1,2,4] Triazole with Different Bicyclic Heteroaromatic Systems’, Eur. J. Med. Chem. 2021, 216, 113331.
- 38J. L. Huo, S. Wang, X. H. Yuan, B. Yu, W. Zhao, H. M. Liu, ‘Discovery Of [1,2,4] Triazolo [1,5-A] Pyrimidines Derivatives as Potential Anticancer Agents’, Eur. J. Med. Chem. 2021, 211, 113108.
- 39M. Mustafa, G. E. D. A. Abuo-Rahma, A. A. A. El-Hafeez, E. R. Ahmed, D. Abdelhamid, P. Ghosh, A. M. Hayallah, ‘Discovery of Antiproliferative and Anti-FAK Inhibitory Activity Of 1,2,4-Triazole Derivatives Containing Acetamido Carboxylic Acid Skeleton’, Bioorg. Med. Chem. Lett. 2021, 40, 127965.
- 40O. Grytsai, O. Valiashko, M. Penco-Campillo, M. Dufies, A. Hagege, L. Demange, S. Martial, G. Pagès, C. Ronco, R. Benhida, ‘Synthesis and Biological Evaluation Of 3-Amino-1,2,4-Triazole Derivatives as Potential Anticancer Compounds’, Bioorg. Chem. 2020, 104, 104271.
- 41S. S. Muzaffar, W. Shahid, N. Riaz, M. Saleem, M. Ashraf, B. Bashir, A. Kaleem, M. Al-Rashida, B. Baral, K. Bhattarai, H. Gross, ‘Probing Phenylcarbamoylazinane-1, 2, 4-Triazole Amide Derivatives as Lipoxygenase Inhibitors Along with Cytotoxic, ADME and Molecular Docking Studies’, Bioorg. Chem. 2021, 107, 104525.
- 42S. Muzaffar, W. Shahid, M. Saleem, M. Ashraf, B. Bashir, M. Ali, M. Al-Rashida, B. Baral, K. Bhattarai, N. Riaz, ‘Evaluation of Ethylated Phenylcarbamoylazinane-1, 2, 4-Triazole Amide Derivatives As 15-Lipoxygenase Inhibitors Together with Cytotoxic, ADME and Molecular Modeling Studies’, ChemistrySelect 2020, 5, 14210–14216.
- 43M. M. Slaihim, F. S. R. Al-Suede, M. Khairuddean, M. B. K. Ahamed, A. M. S. A. Majid, ‘Synthesis, Characterization of New Derivatives with Mono Ring System Of 1,2,4-Triazole Scaffold and Their Anticancer Activities’, J. Mol. Struct. 2019, 1196, 78–87.
- 44A. H. Romero, F. Sojo, F. Arvelo, C. Calderon, A. Morales, S. E. Lopez, ‘Anticancer Potential of New 3-Nitroaryl-6-(N-Methyl) Piperazin-1,2, 4-Triazolo [3, 4-A] Phthalazines Targeting Voltage-Gated K+ Channel: Copper-Catalyzed One-Pot Synthesis From 4-Chloro-1-Phthalazinyl-Arylhydrazones’, Bioorg. Chem. 2020, 101, 104031.
- 45C. Wang, Y. Li, T. Liu, Z. Wang, Y. Zhang, K. Bao, Y. Wu, Q. Guan, D. Zuo, W. Zhang, ‘Design, Synthesis and Evaluation of Antiproliferative and Antitubulin Activities Of 5-Methyl-4-Aryl-3-(4-Arylpiperazine-1-Carbonyl)-4H-1,2, 4-Triazoles’, Bioorg. Chem. 2020, 104, 103909.
- 46S. A. Al-Hussain, T. A. Farghaly, M. E. Zaki, H. G. Abdulwahab, N. T. Al-Qurashi, Z. A. Muhammad, ‘Discovery of Novel Indolyl-1, 2, 4-Triazole Hybrids as Potent Vascular Endothelial Growth Factor Receptor-2 (VEGFR-2) Inhibitors with Potential Anti-Renal Cancer Activity’, Bioorg. Chem. 2020, 105, 104330.
- 47A. T. Boraei, P. K. Singh, M. Sechi, S. Satta, ‘Discovery of Novel Functionalized 1, 2, 4-Triazoles As PARP-1 Inhibitors in Breast Cancer: Design, Synthesis and Antitumor Activity Evaluation’, Eur. J. Med. Chem. 2019, 182, 111621.
- 48F. Naaz, F. Ahmad, B. A. Lone, Y. R. Pokharel, N. K. Fuloria, S. Fuloria, M. Ravichandran, L. Pattabhiraman, S. Shafi, M. S. Yar, ‘Design and Synthesis of Newer 1, 3, 4-Oxadiazole and 1, 2, 4-Triazole Based Topsentin Analogs as Anti-Proliferative Agent Targeting Tubulin’, Bioorg. Chem. 2020, 95, 103519.
- 49H. A. Gomaa, H. A. El-Sherief, S. Hussein, A. M. Gouda, O. I. Salem, K. S. Alharbi, A. M. Hayallah, B. G. Youssif, ‘Novel 1, 2, 4-Triazole Derivatives as Apoptotic Inducers Targeting P53: Synthesis and Antiproliferative Activity’, Bioorg. Chem. 2020, 105, 104369.
- 50A. H. Abdelazeem, A. M. Alqahtani, H. A. Omar, S. N. A. Bukhari, A. M. Gouda, ‘Synthesis, Biological Evaluation and Kinase Profiling of Novel S-Benzo [4, 5] Thiazolo [2,3-C] [1,2,4] Triazole Derivatives as Cytotoxic Agents with Apoptosis-Inducing Activity’, J. Mol. Struct. 2020, 1219, 128567.
- 51E. S. M. Abdelrehim, ‘Synthesis and Screening of New [1, 3, 4] Oxadiazole, [1, 2, 4] Triazole, and [1, 2, 4] Triazolo [4, 3-B] [1, 2, 4] Triazole Derivatives as Potential Antitumor Agents on The Colon Carcinoma Cell Lines (HCT-116)’, ACS Omega 2021, 6, 1687–1696.
- 52A. M. M. Omar, O. M. Aboul-Wafa, M. E. Amr, M. S. El-Shoukrofy, ‘Antiproliferative Activity, Enzymatic Inhibition and Apoptosis-Promoting Effects of Benzoxazole-Based Hybrids on Human Breast Cancer Cells’, Bioorg. Chem. 2021, 109, 104752.
- 53L. Ji, Y. Zhou, Q. Yu, Y. Fang, Y. Jiang, Y. Zhao, C. Yuan, W. Xie, ‘Synthesis and Anticancer Activity of New Spirooxindoles Incorporating [1, 2, 4] Triazolo [3, 4-B] [1, 3, 4] Thiadiazine Moiety’, J. Mol. Struct. 2021, 1227, 129406.
- 54M. Ansari, M. Shokrzadeh, S. Karima, S. Rajaei, S. M. Hashemi, H. Mirzaei, M. Fallah, S. Emami, ‘Design, Synthesis and Biological Evaluation of Flexible and Rigid Analogs Of 4H-1,2,4-Triazoles Bearing 3,4,5-Trimethoxyphenyl Moiety as New Antiproliferative Agents’, Bioorg. Chem. 2019, 93, 103300.
- 55Y. J. Pragathi, R. Sreenivasulu, D. Veronica, R. R. Raju, ‘Design, Synthesis, and Biological Evaluation Of 1, 2, 4-Thiadiazole-1, 2, 4-Triazole Derivatives Bearing Amide Functionality as Anticancer Agents’, Arabian J. Sci. Eng. 2021, 46, 225–232.
- 56D. Kaneko, M. Ninomiya, R. Yoshikawa, Y. Ono, A. D. Sonawane, K. Tanaka, A. Nishina, M. Koketsu, ‘Synthesis Of [1,2,4] Triazolo [4,3-A] Quinoxaline-1,3, 4-Oxadiazole Derivatives as Potent Antiproliferative Agents Via a Hybrid Pharmacophore Approach’, Bioorg. Chem. 2020, 104, 104293.
- 57K. El-Adl, A. G. A. El-Helby, H. Sakr, A. Elwan, ‘Design, Synthesis, Molecular Docking and Anti-Proliferative Evaluations Of [1, 2, 4] Triazolo [4, 3-A] Quinoxaline Derivatives as DNA Intercalators and Topoisomerase II Inhibitors’, Bioorg. Chem. 2020, 105, 104399.
- 58A. M. Mohassab, H. A. Hassan, D. Abdelhamid, A. M. Gouda, B. G. Youssif, H. Tateishi, M. Fujita, M. Otsuka, M. Abdel-Aziz, ‘Design and Synthesis of Novel Quinoline/Chalcone/1, 2, 4-Triazole Hybrids as Potent Antiproliferative Agent Targeting EGFR and BRAFV600E Kinases’, Bioorg. Chem. 2021, 106, 104510.
- 59T. S. Kaoud, A. M. Mohassab, H. A. Hassan, C. Yan, S. X. Van Ravenstein, D. Abdelhamid, K. N. Dalby, M. Abdel-Aziz, ‘NO-Releasing STAT3 Inhibitors Suppress BRAF-Mutant Melanoma Growth’, Eur. J. Med. Chem. 2020, 186, 111885.
- 60H. Wei, Y. Duan, W. Gou, J. Cui, H. Ning, D. Li, Y. Qin, Q. Liu, Y. Li, ‘Design, Synthesis and Biological Evaluation of Novel 4-Anilinoquinazoline Derivatives as Hypoxia-Selective EGFR and VEGFR-2 Dual Inhibitors’, Eur. J. Med. Chem. 2019, 181, 111552.
- 61N. Y. Wang, Y. Xu, K. J. Xiao, W. Q. Zuo, Y. X. Zhu, R. Hu, W. L. Wang, Y. J. Shi, L. T. Yu, Z. H. Liu, ‘Design, Synthesis, and Biological Evaluation Of 4, 5-Dihydro- [1, 2, 4] Triazolo [4,3-F] Pteridine Derivatives as Novel Dual-PLK1/BRD4 Inhibitors’, Eur. J. Med. Chem. 2020, 191, 112152.
- 62I. Shahzadi, A. F. Zahoor, A. Rasul, A. Mansha, S. Ahmad, Z. Raza, ‘Synthesis, Hemolytic Studies, and in silico Modeling of Novel Acefylline-1, 2, 4-Triazole Hybrids as Potential Anti-Cancer Agents Against MCF-7 and A549’, ACS Omega 2021, 6, 11943–11953.
- 63A. Al-Sheikh Ali, D. Khan, A. Naqvi, F. F. Al-Blewi, N. Rezki, M. R. Aouad, M. Hagar, ‘Design, Synthesis, Molecular Modeling, Anticancer Studies, and Density Functional Theory Calculations Of 4-(1, 2, 4-Triazol-3-Ylsulfanylmethyl)-1, 2, 3-Triazole Derivatives’, ACS Omega 2020, 6, 301–316.
- 64L. Gonnet, M. Baron, M. Baltas, ‘Synthesis of Biologically Relevant 1, 2, 3-And 1, 3, 4-Triazoles: From Classical Pathway to Green Chemistry’, Molecules 2021, 26, 5667.
- 65L. Luo, J. J. Jia, Q. Zhong, X. Zhong, S. Zheng, G. Wang, L. He, ‘Synthesis and Anticancer Activity Evaluation of Naphthalene-Substituted Triazole Spirodienones’, Eur. J. Med. Chem. 2021, 213, 113039.
- 66S. M. Chang, V. Jain, T. L. Chen, A. S. Patel, H. B. Pidugu, Y. W. Lin, M. H. Wu, J. R. Huang, H. C. Wu, A. Shah, T. L. Su, ‘Design and Synthesis Of 1, 2-Bis (Hydroxymethyl) Pyrrolo [2, 1-A] Phthalazine Hybrids as Potent Anticancer Agents That Inhibit Angiogenesis and Induce Dna Interstrand Cross-Links’, J. Med. Chem. 2019, 62, 2404–2418.
- 67A. G. A. El-Helby, R. R. Ayyad, H. Sakr, K. El-Adl, M. M. Ali, F. Khedr, ‘Design, Synthesis, Molecular Docking, and Anticancer Activity of Phthalazine Derivatives As VEGFR-2 Inhibitors’, Arch. Pharm. 2017, 350, 1700240.
- 68F. Yang, L. Chen, J. M. Lai, X. E. Jian, D. X. Lv, L. L. Yuan, Y. X. Liu, F. T. Liang, X. L. Zheng, X. L. Li, L. Y. Wei, ‘Synthesis, Biological Evaluation, and Structure-activity Relationship of New Tubulin Polymerization Inhibitors Based On 5-Amino-1, 2, 4-Triazole Scaffold’, Bioorg. Med. Chem. Lett. 2021, 38, 127880.
- 69A. Ammazzalorso, M. Gallorini, M. Fantacuzzi, N. Gambacorta, B. De Filippis, L. Giampietro, C. Maccallini, O. Nicolotti, A. Cataldi, R. Amoroso, ‘Design, Synthesis and Biological Evaluation of Imidazole and Triazole-Based Carbamates as Novel Aromatase Inhibitors’, Eur. J. Med. Chem. 2021, 211, 113115.
- 70X. H. Zhang, H. Q. Kang, Y. Y. Tao, Y. H. Li, J. R. Zhao, L. Y. Ma, H. M. Liu, ‘Identification of Novel 1,3-Diaryl-1,2, 4-Triazole-Capped Histone Deacetylase 6 Inhibitors with Potential Anti-Gastric Cancer Activity’, Eur. J. Med. Chem. 2021, 218, 113392.
- 71M. K. Abdelhameid, I. Zaki, M. R. Mohammed, K. O. Mohamed, ‘Design, Synthesis, and Cytotoxic Screening of Novel Azole Derivatives on Hepatocellular Carcinoma (Hepg2 Cells)’, Bioorg. Chem. 2020, 101, 103995.
- 72S. Akın, E. A. Demir, A. Colak, Y. Kolcuoglu, N. Yildirim, O. Bekircan, ‘Synthesis, Biological Activities and Molecular Docking Studies of Some Novel 2, 4, 5-Trisubstituted-1, 2, 4-Triazole-3-One Derivatives as Potent Tyrosinase Inhibitors’, J. Mol. Struct. 2019, 1175, 280–286.
- 73K. R. Patel, J. G. Brahmbhatt, P. A. Pandya, D. G. Daraji, H. D. Patel, R. M. Rawal, S. K. Baran, ‘Design, Synthesis and Biological Evaluation of Novel 5-(4-Chlorophenyl)-4-Phenyl-4H-1, 2, 4-Triazole-3-Thiols as An Anticancer Agent’, J. Mol. Struct. 2021, 1231, 130000.
- 74S. A. Shahzad, M. Yar, Z. A. Khan, L. Shahzadi, S. A. R. Naqvi, A. Mahmood, S. Ullah, A. J. Shaikh, T. A. Sherazi, A. T. Bale, J. Kukułowicz, ‘Identification Of 1, 2, 4-Triazoles as New Thymidine Phosphorylase Inhibitors: Future Anti-Tumor Drugs’, Bioorg. Chem. 2019, 85, 209–220.
- 75X. Y. Sun, C. Y. Zhong, Q. Q. Qiu, Z. W. Li, M. Y. Liu, X. Wang, C. H. Jin, ‘Synthesis, Activity Evaluation, and Pro-Apoptotic Properties of Novel 1,2,4-Triazol-3-Amine Derivatives as Potent Anti-Lung Cancer Agents’, J. Enzyme Inhib. Med. Chem. 2019, 34, 1210–1217.
- 76D. Chen, Y. Chen, F. Lian, L. Chen, Y. Li, D. Cao, X. Wang, L. Chen, J. Li, T. Meng, M. Huang, ‘Fragment-Based Drug Discovery of Triazole Inhibitors to Block Pdeδ-RAS Protein-Protein Interaction’, Eur. J. Med. Chem. 2019, 163, 597–609.
- 77A. Turky, A. H. Bayoumi, F. F. Sherbiny, K. El-Adl, H. S. Abulkhair, ‘Unravelling the Anticancer Potency Of 1, 2, 4-Triazole-N-Arylamide Hybrids Through Inhibition of STAT3: Synthesis and in silico Mechanistic Studies’, Mol. Diversity 2021, 25, 403–420.
- 78J. Ghanaat, M. A. Khalilzadeh, D. Zareyee, ‘Molecular Docking Studies, Biological Evaluation and Synthesis of Novel 3-Mercapto-1,2,4-Triazole Derivatives’, Mol. Diversity 2021, 25, 223–232.
- 79H. A. Abuelizz, H. M. Awad, M. Marzouk, F. A. Nasr, A. S. Alqahtani, A. H. Bakheit, A. M. Naglah, R. Al-Salahi, ‘Synthesis and Biological Evaluation Of 4-(1H-1,2, 4-Triazol-1-Yl) Benzoic Acid Hybrids as Anticancer Agents’, RSC Adv. 2019, 9, 19065–19074.
- 80Y. Li, Y. He, T. Shao, H. Pei, W. Guo, D. Mi, I. Krimm, Y. Zhang, P. Wang, X. Wang, M. Liu, ‘Modification and Biological Evaluation of a Series Of 1, 5-Diaryl-1, 2, 4-Triazole Compounds as Novel Agents Against Pancreatic Cancer Metastasis Through Targeting Myoferlin’, J. Med. Chem. 2019, 62, 4949–4966.
- 81Z. Wu, X. Li, C. L. Chi, L. Xu, Y. Y. Sun, B. Q. Chen, ‘Synthesis and Antitumor Effects of a New Class Of 1, 2, 4-Triazole Derivatives’, Med. Chem. Res. 2021, 30, 142–151.
- 82N. Li, C. Chen, Z. Shi, J. Sun, L. Chen, ‘Discovery of Novel Celastrol-Triazole Derivatives with Hsp90-Cdc37 Disruption to Induce Tumor Cell Apoptosis’, Bioorg. Chem. 2021, 111, 104867.
- 83A. E. Nofal, I. M. Shatla, D. A. Abdelhafeez, M. Mustafa, O. M. Aly, ‘OMA1520 and OMA1774, Novel 1, 2, 4-Triazole Bearing Analogs of Combretastatin A-4, Inhibit Hepatocellular Carcinoma: Histological and Immunohistochemical Studies’, Biomed. Pharmacother. 2021, 138, 111417.
- 84M. Mustafa, S. Anwar, F. Elgamal, E. R. Ahmed, O. M. Aly, ‘Potent Combretastatin A-4 Analogs Containing 1, 2, 4-Triazole: Synthesis, Antiproliferative, Anti-Tubulin Activity, and Docking Study’, Eur. J. Med. Chem. 2019, 183, 111697.
- 85K. Birgul, Y. Yıldırım, H. Y. Karasulu, E. Karasulu, A. I. Uba, K. Yelekci, H. Bekci, A. Cumaoglu, L. Kabasakal, O. Yılmaz, S. G. Kuçukguzel, ‘Synthesis, Molecular Modeling, in vivo Study and Anticancer Activity Against Prostate Cancer Of (+) (S)-Naproxen Derivatives’, Eur. J. Med. Chem. 2020, 208, 112841.
- 86K. Kumar, R. K. Rawal, ‘Cui/DBU-Mediated MBH Reaction of Isatins: A Convenient Synthesis Of 3-Substituted-3-Hydroxy-2-Oxindole’, ChemistrySelect 2020, 5, 3048–3051.
- 87R. Kaur, K. Kumar, ‘One-Pot Synthesis Of [4-(Tert-Butyl)-1H-Pyrrol-3-Yl] (Phenyl)Methanone from Tosylmethyl Isocyanide and Carbonyl Compound’, Chem. Heterocycl. Compd. 2018, 54, 700–702.
- 88K. Kumar, S. S. More, G. L. Khatik, R. K. Rawal, V. A. Nair, ‘A Highly Stereoselective Chiral Auxiliary-Assisted Reductive Cyclization to Furoindoline’, J. Heterocycl. Chem. 2017, 54, 2696–2702.
- 89K. Kumar, D. Konar, S. Goyal, M. Gangar, M. Chouhan, R. K. Rawal, V. A. Nair, ‘Water Promoted Regiospecific Azidolysis and Copper Catalysed Azide Alkyne Cycloaddition: One-Pot Synthesis Of 3-Hydroxy-1-Alkyl-3-[(4-Aryl/Alkyl-1H-1,2,3-Triazol-1-Yl) Methyl] Indolin-2-One Derivatives’, J. Org. Chem. 2016, 81, 9757–9764.
- 90K. Kumar, J. Siddique, M. Gangar, S. Goyal, R. K. Rawal, V. A. Nair, ‘ZrCl4 Catalysed Diastereoselective Synthesis of Spirocarbocyclic Oxindoles Via [4+2] Cycloaddition’, ChemistrySelect 2016, 1, 2409–2412.
- 91K. Kumar, D. Konar, S. Goyal, M. Gangar, M. Chouhan, R. K. Rawal, V. A. Nair, ‘AlCl3/Cyclohexane Mediated Electrophilic Activation of Isothiocyanates: An Efficient Synthesis of Thioamides’, ChemistrySelect 2016, 1, 3228–3231.
- 92K. Kumar, S. S. More, S. Goyal, M. Gangar, G. L. Khatik, R. K. Rawal, V. A. Nair, ‘A Convenient Synthesis Of 4-Alkyl-3-Benzyolpyrroles From Α, Β-Unsaturated Ketone and Tosylmethylisocyanide’, Tetrahedron Lett. 2016, 57, 2315–2319.
- 93K. Kumar, S. R. Mudshinge, S. Goyal, M. Gangar, V. A. Nair, ‘A Catalyst Free, One Pot Approach for The Synthesis of Quinoxaline Derivatives Via Oxidative Cyclization Of 1,2-Diamines and Phenacyl Bromides’, Tetrahedron Lett. 2015, 56, 1266–1271.
- 94S. Goyal, J. K. Patel, M. Gangar, K. Kumar, V. A. Nair, ‘Zirconocene Dichloride Catalysed One-Pot Synthesis of Pyrroles Through Nitroalkene-Enamine Assembly’, RSC Adv. 2015, 5, 3187–3195.
- 95S. Goyal, B. K. Patel, R. Sharma, M. Chouhan, K. Kumar, M. Gangar, V. A. Nair, ‘An Efficient Strategy for The Synthesis of Syn 1,3-Diols Via Iterative Acetate Aldol Reactions and Synthesis of Atorvastatin Lactone’, Tetrahedron Lett. 2015, 56, 5409–5412.
- 96M. Gangar, N. Kashyap, K. Kumar, S. Goyal, V. A. Nair, ‘Imidazolidinone Based Chiral Auxiliary Mediated Acetate Aldol Reactions of Isatin Derivatives and Stereoselective Synthesis Of 3-Substituted-3-Hydroxy-2-Oxindoles’, Tetrahedron Lett. 2015, 56, 7074–7081.
- 97M. Chouhan, K. R. Senwar, K. Kumar, R. Sharma, V. A. Nair, ‘Catalytic C−H Activation of Arylacetylenes: A Fast Assembly Of 3-Hydroxy-3-(Arylethynyl) Indolin-2-Ones Using Cui/DBU’, Synthesis 2014, 46, 195–202.
- 98V. Kumar, K. Kumar, A. Pal, G. L. Khatik, V. A. Nair, ‘Aldol Reactions Of 2-Thioxotetrahydropyrimidin-4(1H)-Ones: Stereoregulations from Endo- and Exo-Cyclic Chiral Centres’, Tetrahedron 2013, 69, 1747–1754.
- 99R. Sharma, K. Kumar, M. Chouhan, V. Grover, V. A. Nair, ‘Lithium Hydroxide Mediated Synthesis Of 3,4-Disubstituted Pyrroles’, RSC Adv. 2013, 3, 14521–14527.
- 100M. Chouhan, K. Kumar, R. Sharma, V. Grover, V. A. Nair, ‘Nicl2.H2O/Nabh4 in Methanol: A Mild and Efficient Strategy for Chemoselective Deallylation/Debenzylation of Aryl Ethers’, Tetrahedron Lett. 2013, 54, 4540–4543.
- 101K. Kumar, ‘Microwave Assisted Diversified Synthesis of Pyrimidines: An Overview’, J. Heterocycl. Chem. 2022, 59, 205–238.
- 102D. Konar, S. Maru, S. Kar, K. Kumar, ‘Synthesis and Clinical Development of Palbociclib: An Overview’, Med. Chem. 2022, 18, 2–25.
- 103S. Pawar, K. Kumar, M. K. Gupta, R. K. Rawal, ‘Synthetic and Medicinal Perspective of Fused-Thiazoles as Anticancer Agents’, Anti-Cancer Agents Med. Chem. 2021, 21, 1379–1402.
- 104R. Kaur, K. Kumar, ‘Synthetic and Medicinal Perspective of Quinolines as Antiviral Agents’, Eur. J. Med. Chem. 2021, 215, 113220–113258.
- 105K. Kumar, ‘TosMIC: A Powerful Synthon for Cyclization and Sulfonylation’, ChemistrySelect 2020, 5, 10298–10328.
- 106Y. Kapoor, K. Kumar, ‘Structural and Clinical Impact of Anti-Allergy Agents: An Overview’, Bioorg. Chem. 2020, 54, 103351–103375.
- 107R. Kaur, Y. Kapoor, S. K. Manjal, R. K. Rawal, K. Kumar, ‘Diversity-Oriented Synthetic Approaches for Furoindoline: A Review’, Curr. Org. Synth. 2019, 16, 342–368.
- 108S. K. Manjal, S. Pathania, R. Bhatia, R. Kaur, K. Kumar, R. K. Rawal, ‘Diversified Synthetic Strategies for Pyrroloindoles: An Overview’, J. Heterocycl. Chem. 2019, 56, 2318–2332.
- 109R. Kaur, S. K. Manjal, R. K. Rawal, K. Kumar, ‘Recent Synthetic and Medicinal Perspective of Tryptanthrin’, Bioorg. Med. Chem. 2017, 25, 4533–4552.
- 110S. K. Manjal, R. Kaur, R. Bhatia, K. Kumar, V. Singh, R. Shankar, R. Kaur, R. K. Rawal, ‘Synthetic and Medicinal Perspective of Thiazolidinones: A Review’, Bioorg. Chem. 2017, 75, 406–423.
- 111R. Kaur, S. Chaudhary, K. Kumar, M. K. Gupta, R. K. Rawal, ‘Recent Synthetic and Medicinal Perspectives of Dihydropyrimidinones: A Review’, Eur. J. Med. Chem. 2017, 132, 108–134.
- 112B. Kumar, V. Singh, R. Shankar, K. Kumar, R. K. Rawal, ‘Synthetic and Medicinal Prospective of Structurally Modified Curcumins’, Curr. Top. Med. Chem. 2017, 17, 148–161.
- 113M. Mittal, K. Kumar, D. Anghore, R. K. Rawal, ‘ICP-MS: Analytical Method for Identification and Detection of Elemental Impurities’, Curr. Drug Discovery Technol. 2017, 14, 106–120.
- 114P. Talwan, S. Choudhary, K. Kumar, R. K. Rawal, ‘Chemical and Medicinal Versatility of Substituted 1,4-Dihydropyridines’, Curr. Bioact. Compd. 2017, 13, 109–120.
- 115S. Pawar, M. K. Kumawat, M. Kundu, K. Kumar, ‘Synthetic and Medicinal Perspective of Aantileishmanial Agents: An Overview’, J. Mol. Struct. 2023, 1271, 133977.