Organocatalyzed Domino Synthesis of New Thiazole-Based Decahydroacridine-1,8-diones and Dihydropyrido[2,3-d : 6,5-d′]- dipyrimidines in Water as Antimicrobial Agents
Corresponding Author
Manisha R. Bhosle
Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, 431004, Maharashtra India
Search for more papers by this authorSayali A. Kharote
Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, 431004, Maharashtra India
Search for more papers by this authorGiribala M. Bondle
Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, 431004, Maharashtra India
Search for more papers by this authorJaiprakash N. Sangshetti
Department of Pharmaceutical Chemistry, Y. B. Chavan College of Pharmacy, Rafiq Zakaria Campus, Aurangabad, 431001 India
Search for more papers by this authorSiddique A. Ansari
Department of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, Riyadh, P.O. Box 2454, Riyadh, 11451 Saudi Arabia
Search for more papers by this authorHamad M. Alkahtani
Department of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, Riyadh, P.O. Box 2454, Riyadh, 11451 Saudi Arabia
Search for more papers by this authorCorresponding Author
Manisha R. Bhosle
Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, 431004, Maharashtra India
Search for more papers by this authorSayali A. Kharote
Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, 431004, Maharashtra India
Search for more papers by this authorGiribala M. Bondle
Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, 431004, Maharashtra India
Search for more papers by this authorJaiprakash N. Sangshetti
Department of Pharmaceutical Chemistry, Y. B. Chavan College of Pharmacy, Rafiq Zakaria Campus, Aurangabad, 431001 India
Search for more papers by this authorSiddique A. Ansari
Department of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, Riyadh, P.O. Box 2454, Riyadh, 11451 Saudi Arabia
Search for more papers by this authorHamad M. Alkahtani
Department of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, Riyadh, P.O. Box 2454, Riyadh, 11451 Saudi Arabia
Search for more papers by this authorAbstract
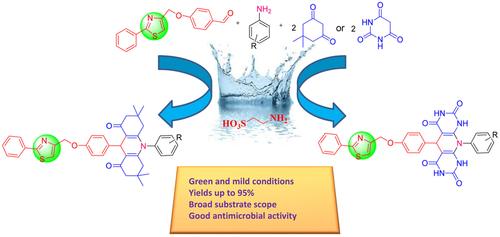
Organopromoter, 2-aminoethanesulfonic acid was used to catalyze the synthesis of a series of structurally intriguing new hybrids thiazolyl acridine-1,8(2H,5H)-diones and dihydropyrido[2,3-d : 6,5-d′]dipyrimidine-2,4,6,8(1H,3H,5H,7H)-tetraones for the first time. 2-Aminoethanesulfonic acid is a biobased organopromoter, used to generate four new bonds for the synthesis of new coupled thiazole-based decahydroacridine-1,8-diones. Superior green credentials, operational simplicity, easy work-up and recyclability of the catalyst are the key strengths of this method. The broad substrate scope, mild reaction conditions, short reaction time, cost effectiveness, high atom economy and good to excellent yields make the present method a distinct improvement over existing methods. Spectral (IR, 1H-NMR,13C-NMR, Mass) data and elemental analyses confirmed the structures of the titled products. A series of thiazolyl acridine-1,8(2H,5H)-diones and dihydropyrido[2,3-d : 6,5-d′]dipyrimidine-2,4,6,8(1H,3H,5H,7H)-tetraones were screened for their antimicrobial activity against four bacterial and three fungal strains.
Graphical Abstract
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv201900577-sup-0001-misc_information.pdf845.8 KB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1I. R. Shaikh, ‘Organocatalysis: Key Trends in Green Synthetic Chemistry, Challenges, Scope towards Heterogenization, and Importance from Research and Industrial Point of View’, J. Catal. 2014, 402860, 1–35.
- 2M. Raj, V. K. Singh, ‘Organocatalytic reactions in water’, Chem. Commun. 2009, 6687–6703.
- 3A. F. de la Torre, A. Ali, F. Z. Galetto, A. L. Braga, J. A. C. Delgado, M. W. Paixao, ‘One-pot organocatalytic/multicomponent approach for the preparation of novel enantioenriched non-natural selenium-based peptoids and peptide-peptoid conjugates’, Mol. Diversity 2019, https://doi.org/ 10.1007/s11030-019-09923-w.
- 4C. M. Marson, ‘Multicomponent and sequential organocatalytic reactions: diversity with atom-economy and enantiocontrol’, Chem. Soc. Rev. 2012, 41, 7712–7722.
- 5R. Ramesh, J. Jayamathi, C. Karthika, J. G. Malecki, A. Lalitha, ‘Innovative Green Synthesis of 4-Aryl-Pyrazolo[5,6]Pyrano[2,3-d]Pyrimidines under Catalyst-Free Conditions’, Polycycl. Arom. Comp. 2018 https://doi.org/ 10.1080/10406638.2018.1454968.
- 6P. Dalko, L. Moisan, ‘Enantioselective Organocatalysis’, Angew. Chem. Int. Ed. 2001, 40, 3726–3748; Angew. Chem. 2001, 113, 3840–3864.
- 7D. Enders, O. Niemeier, A. Henseler, ‘Organocatalysis by N-Heterocyclic Carbenes’, Chem. Rev. 2007, 107, 5606–5655.
- 8A. Dondoni, A. Massi, ‘Asymmetric Organocatalysis: From Infancy to Adolescence’, Angew. Chem. 2008, 47, 4638–4660.
- 9C. M. Volla, I. Atodiresei, M. Rueping, ‘Catalytic C−C Bond-Forming Multi-Component Cascade or Domino Reactions: Pushing the Boundaries of Complexity in Asymmetric Organocatalysis’, Chem. Rev. 2014, 114, 2390–2431.
- 10F. Nafiseh, A. R. Sardarian, ‘Citric Acid: A Green Bioorganic Catalyst for One-Pot Three-Component Synthesis of 2,3-dihydroquinazoline-4(1H)-ones’, Curr. Organocatal. 2016, 3, 39–44.
- 11S. Caputo, L. Banfi, A. Basso, A. Galatini, L. Moni, R. Riva, C. Lambruschini, ‘Diversity-Oriented Synthesis of Various Enantiopure Heterocycles by Coupling Organocatalysis with Multicomponent Reactions’, Eur. J. Org. Chem. 2017, 6619–6628.
- 12H. Kataoka, N. Ohnishi, ‘Occurrence of Taurine in Plants’, Agric. Biol. Chem. 1986, 50, 1887–1888.
- 13G. P. Salz, D. A. Davis, ‘Taurine: a critical nutrient for future fish feeds’, Aquaculture 2015, 437, 215–229.
- 14J. M. Menzie, C. Pan, H. Prentice, J. Y. Wu, ‘Taurine and central nervous system disorders’, Amino Acids 2014, 46, 31–46.
- 15F. Shirini, N. Daneshvar, ‘Introduction of taurine (2-aminoethanesulfonic acid) as a green bio-organic catalyst for the promotion of organic reactions under green conditions’, RSC Adv. 2016, 6, 110190–110205.
- 16N. Daneshvar, F. Shirini, M. Safarpoor, N. Langarudi, R. Karimi-Chayjani, ‘Taurine as a green bio-organic catalyst for the preparation of bio-active barbituric and thiobarbituric acid derivatives in water media’, Bioorg. Chem. 2018, 77, 68–73.
- 17H. M. Shen, H. B. Ji, H. X. Shi, Y. B. She, W. J. Zhou, H. K. Wu, W. B. Yu, N. Ai, ‘Metal-free chemoselective oxidation of sulfides to sulfoxides catalyzed by immobilized taurine and homotaurine in aqueous phase at room temperature’, Tetrahedron Lett. 2015, 56, 4494.
- 18O. Tabarrini, G. Manfroni, A. Fravolini, V. Cecchetti, S. Sabatini, E. De Clercq, J. Rozenski, B. Canard, H. Dutartre, J. Paeshuyse, J. Neyts, ‘Synthesis and Anti-BVDV Activity of Acridones As New Potential Antiviral Agents’, J. Med. Chem. 2006, 49, 2621–2627.
- 19M. M. Amini, Y. Fazaeli, Z. Yassaee, S. Feizi, A. Bazgir, ‘Polytungstozincate acid: a new and efficient catalyst for the synthesis of xanthenes under solvent-free conditions’, Open Catal. J. 2009, 2, 40–44.
- 20M. G. Kowalewska, G. Cholewiński, K. Dzierzbicka, ‘Recent developments in the synthesis and biological activity of acridine/acridone analogs’, RSC Adv. 2017, 7, 15776–15804.
- 21J. P. Poupelin, G. Saint-Rut, O. Foussard-Blanpin, G. Narcisse, G. Uchida-Ernouf, R. Lacroix, ‘Synthesis and Anti-inflammatory Properties of Bis(2-Hydroxy, 1-Naphthyl) Methane Derivatives’, Eur. J. Med. Chem. 1978, 13, 67–71.
- 22R. M. Ion, D. Frackowiak, A. Planner, K. Wiktorowicz, ‘The incorporation of various porphyrins into blood cells measured via flow cytometry, absorption and emission spectroscopy’, Acta Biochim. Pol. 1998, 45, 833–845.
- 23R. J. Harrison, J. Cuesta, G. Chessari, M. A. Read, S. K. Basra, A. P. Reszka, J. Morrell, S. M. Gowan, C. M. Incles, F. A. Tanious, W. D. Wilson, L. R. Kelland, S. Neidle, ‘Trisubstituted acridine derivatives as potent and selective telomerase inhibitors’, J. Med. Chem. 2003, 46, 4463–4476.
- 24T. E. Glotova, M. Y. Dvorko, A. I. Albanov, O. N. Kazheva, G. V. Shilov, O. A. D'yachenko, ‘1,3-Dipolar cycloaddition of 3-phenylamino-5-phenylimino-1,2,4-dithiazole to 1-acyl-2-phenylacetylenes – A new route to functionalized 1,3-thiazole derivatives’, Russ. J. Org. Chem. 2008, 44, 1532–1537.
- 25R. K. Yadlapalli, O. P. Chourasia, M. P. Jogi, A. R. Podile, R. S. Perali, ‘Design, synthesis and in vitro antimicrobial activity of novel phenylbenzamido-aminothiazole-based azasterol mimics’, Med. Chem. Res. 2013, 22, 2975–2983.
- 26T. I. de Santana, M. de O. Barbosa, P. Andr, T. de M. Gomes, A. C. N. da Cruz, T. G. da Silva, A. C. L. Leite, ‘Synthesis, anticancer activity and mechanism of action of new thiazole derivatives’, Eur. J. Med. Chem. 2018, 144, 874–886.
- 27W. Xie, Y. Wu, J. Zhang, Q. Mei, Y. Zhang, N. Zhu, R. Liu, H. Zhang, ‘Design, synthesis and biological evaluations of novel pyridone-thiazole hybrid molecules as antitumor agents’, Eur. J. Med. Chem. 2018, 145, 35–40.
- 28A. Rouf, C. Tanyeli, ‘Bioactive thiazole and benzothiazole derivatives’, Eur. J. Med. Chem. 2015, 97, 911–927.
- 29C. Chen, J. Song, J. Wanga, C. Xu, C. Chen, W. Gu, H. Sun, X. Wena, ‘Synthesis and biological evaluation of thiazole derivatives as novel USP7 inhibitors’, Bioorg. Med. Chem. Lett. 2017, 27, 845–849.
- 30M. Kiani, M. Mohammadipour, ‘Fe3O4@SiO2−MoO3H nanoparticles: a magnetically recyclable nanocatalyst system for the synthesis of 1,8-dioxo-decahydroacridine derivatives’, RSC Adv. 2017, 7, 997–1007.
- 31B. Aday, Y. Yıldız, R. Ulus, S. Eris, F. Sen, M. Kaya, ‘One-pot, efficient and green synthesis of acridinedione derivatives using highly monodisperse platinum nanoparticles supported with reduced graphene oxide’, New J. Chem. 2016, 40, 748–754.
- 32K. Venkatesan, S. S. Pujari, K. V. Srinivasan, ‘Proline-catalyzed simple and efficient synthesis of 1,8-dioxo-decahydroacridines in aqueous ethanol medium’, Synth. Commun. 2009, 39, 228–241.
- 33X. S. Wang, D. Q. Shi, D. Q. Zhang, Y. F. Wang, S. J. Tu, ‘Synthesis of 9-arylpolyhydroacridine in water catalyzed by triethylbenzylammonium chloride (TEBA)’, Chin. J. Chem. 2004, 24, 430–432.
- 34W. Shen, L. M. Wang, H. Tian, J. Tang, J. J. Yu, ‘Brønsted acidic imidazolium salts containing perfluoroalkyl tails catalyzed one-pot synthesis of 1,8-dioxo-decahydroacridines in water’, J. Fluorine Chem. 2009, 130, 522–527.
- 35M. Dabiri, M. Baghbanzadeh, E. Arzroomchilar, ‘1-Methylimidazolium triflouroacetate ([Hmim]TFA): An efficient reusable acidic ionic liquid for the synthesis of 1,8-dioxo-octahydroxanthenes and 1,8-dioxo-decahydroacridines’, Catal. Commun. 2008, 9, 939–942.
- 36M. Kidwai, D. Bhatnagar, ‘Ceric ammonium nitrate (CAN) catalyzed synthesis of N-substituted decahydroacridine-1,8-diones in PEG’, Tetrahedron Lett. 2010, 51, 2700–2703.
- 37F. Rashedian, D. Saberib, K. Niknam, ‘Silica-Bonded N-Propyl Sulfamic Acid: A Recyclable Catalyst for the Synthesis of 1,8-Dioxo-decahydroacridines, 1,8-Dioxo-octahydroxanthenes and Quinoxalines’, J. Chin. Chem. Soc. 2010, 57, 998–1006.
- 38B. Das, P. Thirupathi, I. Mahender, V. S. Reddy, Y. K. Rao, ‘Amberlyst-15: An Efficient Reusable Heterogeneous Catalyst for the Synthesis of 1,8-Dioxo-Octahydroxanthenes and 1,8-Dioxo-Decahydroacridinesao’, J. Mol. Catal. A 2006, 247, 233–239.
- 39A. Davoodnia, A. Khojastehnezhad, N. Tavakoli-Hoseini, ‘Carbon-based solid acid as an efficient and reusable catalyst for the synthesis of 1,8-dioxodecahydroacridines under solvent-free conditions’, Bull. Korean Chem. Soc. 2011, 32, 2243–2248.
- 40T. S. Jin, J. S. Zhang, T. T. Guo, A. Q. Wang, T. S. Li, ‘One-pot clean synthesis of 1,8-dioxo-decahydroacridines catalyzed by p-dodecylbenezenesulfonic acid in aqueous media’, Synthesis 2004, 12, 2001–2005.
- 41A. Işık, B. Aday, R. Ulus, M. Kaya, ‘One-Pot, Facile, Highly Efficient, and Green Synthesis of Acridinedione Derivatives Using Vitamin B1’, Synth. Commun. 2015, 1, 2823–2831.
- 42N. Hazeri, A. Masoumnia, M. Taher, M. S. Salahi, M. Kangani, S. Kianpour, S. Kiaee, J. Abonajmi, ‘Acetic acid as an efficient catalyst for synthesis of 1,8-dioxo-octahydroxanthenes and 1,8-dioxo-decahydroacridines’, Res. Chem. Intermed. 2015, 41, 4123–4131.
- 43M. R. Bhosle, P. Andil, D. Wahul, G. M. Bondle, A. Sarkate, S. V. Tiwari, ‘Straightforward multicomponent synthesis of pyrano[2,3-d]pyrimidine-2,4,7-triones in β-cyclodextrin cavity and evaluation of their anticancer activity’, J. Iran. Chem. Soc. 2019, 16, 1553–1561.
- 44M. R. Bhosle, D. Nipte, J. Gaikwad, M. A. Shaikh, G. M. Bondle, J. N. Sangshetti, ‘A rapid and green method for expedient multicomponent synthesis of N-substituted decahydroacridine-1,8-diones as potential antimicrobial agents’, Res. Chem. Intermed. 2018, 44, 7047–7064.
- 45C. Jadhav, L. D. Khillare, M. R. Bhosle, ‘Efficient sonochemical protocol for the facile synthesis of dipyrimido-dihydropyridine and pyrimido[4,5-d]pyrimidines in aqueous β-cyclodextrin’, Synth. Commun. 2018, 48, 233–246.
- 46M. R. Bhosle, L. D. Khillare, J. R. Mali, A. P. Sarkate, D. K. Lokwani, S. V. Tiwarie, ‘DIPEAc promoted one-pot synthesis of dihydropyrido[2,3-d : 6,5-d′]dipyrimidinetetraone and pyrimido[4,5-d]pyrimidine derivatives as potent tyrosinase inhibitors’, New J. Chem. 2018, 42, 18621.
- 47M. R. Bhosle, J. R. Mali, U. R. Pratap, R. A. Mane, ‘An Efficient Synthesis of New Pyrazolines and Isoxazolines Bearing Thiazolyl and Etheral Pharmacophores’, Bull. Korean Chem. Soc. 2012, 33, 2012–2016.
- 48Y. Gu, ‘Multicomponent reactions in unconventional solvents: state of the art’, Green Chem. 2012, 14, 2091–2128.
- 49S. K. Rout, S. Guin, J. Nath, B. K. Patel, ‘An ‘on-water’ exploration of CuO nanoparticle catalysed synthesis of 2-aminobenzothiazoles’, Green Chem. 2012, 14, 2491–2498.
- 50C.-J. Li, L. Chen, ‘Organic chemistry in water’, Chem. Soc. Rev. 2006, 35, 68–82.