Breaking Down the Barriers to a Natural Antiviral Agent: Antiviral Activity and Molecular Docking of Erythrina speciosa Extract, Fractions, and the Major Compound
Nouran M. Fahmy
Department of Pharmacognosy, Faculty of Pharmacy, Ain-Shams University, Abassia, 11566 Cairo, Egypt
Both authors equally contributed to this work.
Search for more papers by this authorEman Al-Sayed
Department of Pharmacognosy, Faculty of Pharmacy, Ain-Shams University, Abassia, 11566 Cairo, Egypt
Both authors equally contributed to this work.
Search for more papers by this authorSaad Moghannem
Department of Botany and Microbiology, Faculty of Science, Al-Azhar University, 11884 Cairo, Egypt
Search for more papers by this authorCorresponding Author
Faizul Azam
Department of Pharmaceutical Chemistry and Pharmacognosy, Unaizah College of Pharmacy, Qassim University, 51911 Unaizah, Saudi Arabia
Search for more papers by this authorCorresponding Author
Mohamed El-Shazly
Department of Pharmacognosy, Faculty of Pharmacy, Ain-Shams University, Abassia, 11566 Cairo, Egypt
Department of Pharmaceutical Biology, Faculty of Pharmacy and Biotechnology, German University in Cairo, 11835 Cairo, Egypt
Search for more papers by this authorCorresponding Author
Abdel Nasser Singab
Department of Pharmacognosy, Faculty of Pharmacy, Ain-Shams University, Abassia, 11566 Cairo, Egypt
Center for Drug Discovery and Development Research, Faculty of Pharmacy, Ain Shams University, 11566 Cairo, Egypt
Search for more papers by this authorNouran M. Fahmy
Department of Pharmacognosy, Faculty of Pharmacy, Ain-Shams University, Abassia, 11566 Cairo, Egypt
Both authors equally contributed to this work.
Search for more papers by this authorEman Al-Sayed
Department of Pharmacognosy, Faculty of Pharmacy, Ain-Shams University, Abassia, 11566 Cairo, Egypt
Both authors equally contributed to this work.
Search for more papers by this authorSaad Moghannem
Department of Botany and Microbiology, Faculty of Science, Al-Azhar University, 11884 Cairo, Egypt
Search for more papers by this authorCorresponding Author
Faizul Azam
Department of Pharmaceutical Chemistry and Pharmacognosy, Unaizah College of Pharmacy, Qassim University, 51911 Unaizah, Saudi Arabia
Search for more papers by this authorCorresponding Author
Mohamed El-Shazly
Department of Pharmacognosy, Faculty of Pharmacy, Ain-Shams University, Abassia, 11566 Cairo, Egypt
Department of Pharmaceutical Biology, Faculty of Pharmacy and Biotechnology, German University in Cairo, 11835 Cairo, Egypt
Search for more papers by this authorCorresponding Author
Abdel Nasser Singab
Department of Pharmacognosy, Faculty of Pharmacy, Ain-Shams University, Abassia, 11566 Cairo, Egypt
Center for Drug Discovery and Development Research, Faculty of Pharmacy, Ain Shams University, 11566 Cairo, Egypt
Search for more papers by this authorAbstract
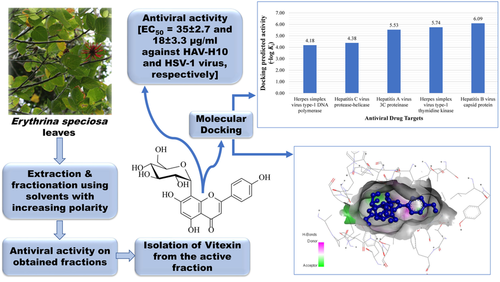
The in vitro cytotoxic activity in Vero cells and the antiviral activity of Erythrina speciosa methanol extract, fractions, and isolated vitexin were studied. The results revealed that E. speciosa leaves ethyl acetate soluble fraction of the methanol extract (ESLE) was the most active against herpes simplex virus type 1 (HSV-1). Bioactivity-guided fractionation was performed on ESLE to isolate the bioactive compounds responsible for this activity. One sub-fraction from ESLE (ESLE IV) showed the highest activity against HSV-1 and Hepatitis A HAV-H10 viruses. Vitexin isolated from ESLE VI exhibited a significant antiviral activity (EC50=35±2.7 and 18±3.3 μg/mL against HAV-H10 and HSV-1 virus, respectively), which was notably greater than the activity of the extract and the fractions. Molecular docking studies were carried out to explore the molecular interactions of vitexin with different macromolecular targets. Analysis of the in silico data together with the in vitro studies validated the antiviral activity associated with vitexin. These outcomes indicated that vitexin is a potential candidate to be utilized commendably in lead optimization for the development of antiviral agents.
Graphical Abstract
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv201900511-sup-0001-misc_information.pdf296.7 KB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1K. Zandi, E. Ramedani, K. Mohammadi, S. Tajbakhsh, I. Deilami, Z. Rastian, M. Fouladvand, F. Yousefi, F. Farshadpour, ‘Evaluation of Antiviral Activities of Curcumin Derivatives against HSV-1 in Vero Cell Line’, Nat. Prod. Commun. 2010, 5, 1935–1938.
- 2L.-T. Lin, W.-C. Hsu, C.-C. Lin, ‘Antiviral Natural Products and Herbal Medicines’, J. Tradit. Med. 2014, 4, 24–35.
- 3G. McQuillan, D. Kruszon-Moran, E. W. Flagg, R. Paulose-Ram, ‘Prevalence of Herpes Simplex Virus Type 1 and Type 2 in Persons aged 14–49: United States, 2015–2016’, NCHS Data Brief., 2018, Vol. 304. Hyattsville, MD, National Center for Health Statistics.
- 4É. Benassi-Zanqueta, C. F. Marques, L. M. Valone, B. L. Pellegrini, A. Bauermeister, I. C. P. Ferreira, N. P. Lopes, C. V. Nakamura, B. P. Dias Filho, M. R. M. Natali, T. Ueda-Nakamura, ‘Evaluation of anti-HSV-1 activity and toxicity of hydroethanolic extract of Tanacetum parthenium (L.) Sch. Bip.(Asteraceae)’, Phytomedicine 2019, 55, 249–254.
- 5C. D. Conrady, D. A. Drevets, D. J. J. Carr, ‘Herpes simplex type I (HSV-1) infection of the nervous system: Is an immune response a good thing?’, J. Neuroimmunol. 2010, 220, 1–9.
- 6S. P. Kouyoumjian, H. Chemaitelly, L. J. Abu-Raddad, ‘Characterizing hepatitis C virus epidemiology in Egypt: systematic reviews, meta-analyses, and meta-regressions’, Sci. Rep. 2018, 8, 1661.
- 7J. D. Stanaway, A. D. Flaxman, M. Naghavi, C. Fitzmaurice, T. Vos, I. Abubakar, L. J. Abu-Raddad, R. Assadi, N. Bhala, B. Cowie, M. H. Forouzanfour, J. Groeger, K. M. Hanafiah, K. H. Jacobsen, S. L. James, J. MacLachlan, R. Malekzadeh, N. K. Martin, A. A. Mokdad, A. H. Mokdad, C. J. L. Murray, D. Plass, S. Rana, D. B. Rein, J. H. Richardus, J. Sanabria, M. Saylan, S. Shahraz, S. So, V. V. Vlassov, E. Weiderpass, S. T. Wiersma, M. Younis, C. Yu, M. El Sayed Zaki, G. S. Cooke, ‘The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013’, Lancet 2016, 388, 1081–1088.
- 8L. S. Y. Tang, E. Covert, E. Wilson, S. Kottilil, ‘Chronic Hepatitis B Infection: A Review’, J. Am. Med. Women′s Assoc. 2018, 319, 1802–1813.
- 9D. J. Seo, M. Lee, S. B. Jeon, H. Park, S. Jeong, B.-H. Lee, C. Choi, ‘Antiviral activity of herbal extracts against the hepatitis A virus’, Food Control 2017, 72, 9–13.
- 10T. Li, T. Peng, ‘Traditional Chinese herbal medicine as a source of molecules with antiviral activity’, Antiviral Res. 2013, 97, 1–9.
- 11M. M. Cowan, ‘Plant Products as Antimicrobial Agents’, Clin. Microbiol. Rev. 1999, 12, 564–582.
- 12N. M. Fahmy, E. Al-Sayed, M. El-Shazly, A. N. Singab, ‘Alkaloids of genus Erythrina: An updated review’, Nat. Prod. Res. 2019, 1–22.
- 13N. M. Fahmy, E. Al-Sayed, M. El-Shazly, A. N. Singab, ‘Comprehensive review on flavonoids biological activities of Erythrina plant species’, Ind. Crops Prod. 2018, 123, 500–538.
- 14P.-R. Suley, J. L. González-Guevara, M. García-Torres, M. T. Carballo-González, O. A. Echemendia-Arana, G. Garrido-Garrido, J. A. González-Lavaut, J. Molina-Torres, S. Prieto-González, ‘Preliminary Phytochemical Screening and In Vitro Antiherpetic Activity of Erythrina fusca Lour.’, Acta Farm. Bonaerense 2004, 23, 453–458.
- 15A. K. Wardani, A. Mun'im, A. Yanuar, ‘Inhibition of HIV-1 Reverse Transcriptase of Selected Indonesia Medicinal Plants and Isolation of the Inhibitor from Erythrina variegata L. Leaves’, J. Young Pharm. 2018, 10, 169–172.
- 16T. N. Kaul, E. Middleton Jr., P. L. Ogra, ‘Antiviral effect of flavonoids on human viruses’, J. Med. Virol. 1985, 15, 71–79.
- 17P. Wen, H. Han, R. Wang, N. Wang, X. Yao, ‘C-glycosylfavones and aromatic glycosides from Campylotropis hirtella (Franch.) Schindl’, Asian J. Tradit. Med. 2007, 2, 149–153.
- 18S. Rayyan, T. Fossen, Ø. M. Andersen, ‘Flavone C-Glycosides from Seeds of Fenugreek, Trigonella foenum-graecum L.’, J. Agric. Food Chem. 2010, 58, 7211–7217.
- 19T. J. Mabry, K. R. Markham, M. B. Thomas, ‘The Systematic Identification of Flavonoids’, Springer-Verlag, Berlin Heidelberg, 1970.
- 20N. M. Fahmy, E. Al-Sayed, M. M. Abdel-Daim, A. N. Singab, ‘Anti-Inflammatory and Analgesic Activities of Terminalia Muelleri Benth.(Combretaceae)’, Drug Dev. Res. 2017, 78, 146–154.
- 21A. B. Barakat, S. A. Shoman, N. Dina, O. R. Alfarouk, ‘Antiviral activity and mode of action of Dianthus caryophyllus L. and Lupinus termes L. seed extracts against In Vitro herpes simplex and hepatitis A viruses infection’, J. Microbiol. 2010, 2, 23–29.
- 22S. T. Hassan, M. Šudomová, K. Berchová-Bímová, K. Šmejkal, J. Echeverría, ‘Psoromic Acid, a Lichen-Derived Molecule, Inhibits the Replication of HSV-1 and HSV-2, and Inactivates HSV-1 DNA Polymerase: Shedding Light on Antiherpetic Properties’, Molecules 2019, 24, 2912.
- 23H. A. M. Goma′a, M. A. Ghaly, L. A. Abou-zeid, F. A. Badria, I. A. Shehata, M. M. El-Kerdawy, ‘Synthesis, Biological Evaluation and in silico Studies of 1,2,4-Triazole and 1,3,4-Thiadiazole Derivatives as Antiherpetic Agents’, Chem. Select 2019, 4, 6421–6428.
10.1002/slct.201900814 Google Scholar
- 24A. F. Kassem, R. Z. Batran, E. M. Abbas, S. A. Elseginy, M. N. F. Shaheen, E. M. Elmahdy, ‘New 4-phenylcoumarin derivatives as potent 3 C protease inhibitors: Design, synthesis, anti-HAV effect and molecular modeling’, Eur. J. Med. Chem. 2019, 168, 447–460.
- 25L. T. Song, R. R. Liu, H. L. Zhai, Y. J. Meng, M. Zhu, ‘Molecular mechanisms of tetrahydropyrrolo[1,2-c]pyrimidines as HBV capsid assembly inhibitors’, Arch. Biochem. Biophys. 2019, 663, 1–10.
- 26A. K. Lampa, S. M. Bergman, S. S. Gustafsson, H. Alogheli, E. B. Åkerblom, G. G. Lindeberg, R. M. Svensson, P. Artursson, U. H. Danielson, A. Karlén, A. Sandström, ‘Novel Peptidomimetic Hepatitis C Virus NS3/4 A Protease Inhibitors Spanning the P2–P1′ Region’, ACS Med. Chem. Lett. 2013, 5, 249–254.
- 27E. E. M. Eid, F. Azam, M. Hassan, I. M. Taban, M. A. Halim, ‘Zerumbone binding to estrogen receptors: an in-silico investigation’, J. Recept. Signal Transduction 2018, 38, 342–351.
- 28F. Azam, N. H. Alabdullah, H. M. Ehmedat, A. R. Abulifa, I. Taban, S. Upadhyayula, ‘NSAIDs as potential treatment option for preventing amyloid β toxicity in Alzheimer's disease: an investigation by docking, molecular dynamics, and DFT studies’, J. Biomol. Struct. Dyn. 2018, 36, 2099–2117.
- 29F. Azam, A. M. Amer, A. R. Abulifa, M. M. Elzwawi, ‘Ginger components as new leads for the design and development of novel multi-targeted anti-Alzheimer's drugs: a computational investigation’, Drug Des. Dev. Ther. 2014, 8, 2045–2059.
- 30M. Attia, K. Abdel-Wahab, R. Arafa, M. Awadallah, ‘Saliva versus serum for serodiagnosis of herpes simple virus (HSV) infection in apparently healthy individuals’, Al-Azhar J. Microbiol. 1991, 13, 130–138.
- 31M. Ali, K. Abdel-Wahab, ‘Isolation of hepatitis A virus from stools and the development of a dot ELISA’, J. Trop. Med. Hyg. 1991, 15, 35–44.
- 32L. J. Reed, H. Muench, ‘A simple method of estimating fifty percent endpoints’, Am. J. Epidemiol. 1938, 27, 493–497.
10.1093/oxfordjournals.aje.a118408 Google Scholar
- 33P. Sethi, ‘Activity of Turbinaria ornata (Turner) J. Agade against Blue Tongue Virus (Btv)’, IOSR J. Pharm. 2016, 6, 93–95.
- 34G. M. Morris, D. S. Goodsell, R. Huey, A. J. Olson, ‘Distributed automated docking of flexible ligands to proteins: parallel applications of AutoDock 2.4’, J. Comput.-Aided Mol. Des. 1996, 10, 293–304.