Meta-Analysis of the Relationship Between Occupational/Environmental Exposure to Wood Dust and Laryngeal Cancer
Funding: This work was supported by Jiangsu Province Occupational Health Research Project (JSZJ20231209).
Zhou Xin and Dou Jianrui contributed equally to this study and are the co-first authors of this article.
ABSTRACT
Objective
Wood dust is a human carcinogen. However, studies examining the relationship between wood dust exposure and laryngeal cancer have yielded inconsistent findings. Therefore, we systematically reviewed relevant studies examining the relationship between wood dust exposure and laryngeal cancer development, followed by a meta-analysis.
Methods
Publications in the following databases were searched: PubMed, Medline, Embase, Cochrane Library, and China National Knowledge Infrastructure (CNKI). The Newcastle–Ottawa scale was used to evaluate the study quality. A random-effects model was used for the meta-analysis.
Results
Eighteen case–control studies and one cohort study, involving a total of 4426 patients with laryngeal cancer and 319,129 control participants, were identified. The association between occupational/environmental exposure to wood dust and laryngeal cancer, if any, was unclear (adjusted combined OR: 1.11; 95% CI: 0.94–1.31). However, subgroup analyses according to the number of cases, geographic region, publication year, and follow-up duration revealed correlations between wood dust exposure correlated and laryngeal cancer, as follows: number of cases > 200 (OR: 1.14; 95% CI: 1.01–1.25 [n = 10]); studies conducted in the US (OR: 1.21; 95% CI: 1.07–1.37 [n = 5]); follow-up time > 5 years (OR: 1.19; 95% CI: 1.07–1.32 [n = 10]); and publication after the year 2000 (OR: 1.15; 95% CI: 1.04–1.28 [n = 8]). A high heterogeneity in the results was observed (I2 = 42.5%, p = 0.024). The results were stable, and no publication bias existed, according to sensitivity analysis.
Conclusions
This meta-analysis suggests that wood dust exposure is associated with laryngeal cancer. Additional large-scale studies are warranted to clarify the relationship between wood dust exposure and laryngeal cancer.
1 Introduction
Wood dust is a harmful by-product widely present in wood-processing industries such as sawmills, furniture manufacturing, and cupboard production [1]. The International Agency for Research on Cancer (IARC) classified wood dust as a class I carcinogen in 2009. Specifically, wood dust can cause nasal cavity, paranasal sinus, and nasopharynx cancer [2]. Notably, workers in a wide range of jobs are exposed to wood dust worldwide [3]. Approximately 1.7 million workers in the UK are exposed to low (< 0.5 mg/m3) or high (> 5 mg/m3) levels of wood dust [4]. Moreover, 2.3% of workers in Finland [5], 1.3% of workers in Italy [6], and 1.8% of workers in France [7] have been exposed to wood dust. The highest cumulative wood dust exposure is 28.82 mg/m3-y in four Nordic countries, and many workers have been exposed to wood dust for > 10 years [8]. In France, approximately 75 cases of occupational disease after exposure to wood dust have been reported every year, and compensation of 20–30 million euros is paid to employees [9].
Exposure to wood dust can lead to several health problems, particularly respiratory tract cancer. A meta-analysis has indicated that exposure to wood dust has a stronger association with nasal adenocarcinoma than lung cancer [10]. Our previous study confirmed that wood dust exposure poses a dose-dependent risk of nasopharyngeal carcinoma [11] (OR: 2.18; 95% CI: 1.62–2.93). However, studies on the association between wood dust exposure and laryngeal cancer have shown widely varying results and have yielded no definitive findings regarding the association. Some studies have reported wood dust exposure as a potential risk factor for laryngeal cancer [12-20], some have demonstrated an association [21-23], whereas others have found no association [24-30]. A recent meta-analysis by Paget-Bailly, Cyr, and Luce [31] has concluded no association between wood dust exposure and the risk of laryngeal cancer. Notably, that meta-analysis [31] included studies published until 2012, and the association between wood dust exposure and laryngeal cancer in some studies was analyzed according to the standardized mortality ratio. Many cases included in these studies were not original cases, and the sample sizes were small. The populations in Wortley's et al. 1992 study [24] and Vaughan and Davis 1991 study [32] were from the same case–control study. Because the evidence linking wood dust exposure to laryngeal cancer risk is inconclusive, the objective of the current meta-analysis was to increase the study sample size and to investigate the potential association, if any, through a case–control design.
2 Materials and Methods
This meta-analysis was based strictly on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [33] and Cochrane Collaboration guidelines [34].
2.1 Search Strategy
We searched the PubMed, Embase, Medline, Cochrane Library, and China National Knowledge Infrastructure (CNKI) databases without language restrictions. The search for original manuscripts was conducted on database records from January 1991 through June 2024. To search for additional studies, we also manually searched the references of the included studies.
Search strings included the following indexing terms: wood dust (“wood dust,” “sawdust,” “saw dust,” “hardwood dust,” and “softwood dust”); and laryngeal cancer (“laryngeal cancer,” “larynx cancer,” “laryngeal carcinoma,” and “laryngeal malignancy”). No additional restrictions were used in the search.
2.2 Study Selection and Eligibility Criteria
The inclusion criteria were as follows: (1) cohort or case–control study design; (2) reporting of the role of occupational/environmental exposure to wood dust in laryngeal cancer, including the effect size (odds ratio [OR] and relative risk) and confidence interval (CI), with adjustment for confounding factors; (3) control group comprising individuals with low or no exposure to wood dust; (4) confirmation of laryngeal cancer through clinical diagnosis in a hospital (International Classification of Diseases for Oncology [ICD-O]); and (5) original reports.
The exclusion criteria were as follows: (1) studies not pertaining to wood dust exposure and laryngeal cancer; (2) studies with insufficient data; (3) case reports, reviews, and commentaries; and (4) cross-sectional studies.
Additionally, only studies with relatively large sample sizes were selected to ensure study independence if the data were used in more than one study or if one study included findings from multiple studies.
2.3 Data Extraction
Two reviewers (Meng and Jinzhu) independently assessed the titles and abstracts of the selected studies and compared the data separately to ensure data completeness and accuracy. The following data were extracted: author; date of publication; study year(s); study type; number of cases and controls; geographic location where the study was conducted; OR; 95% CI; and adjustment factors. Any inconsistencies in data extraction were resolved through discussion with a third reviewer.
2.4 Quality Assessment
The quality of the selected studies was assessed with the Newcastle–Ottawa scale [35], comprising nine items, each assigned a value of 1 point. The study quality assessment was independently performed by two authors, and inconsistent scores were resolved through discussion. Only scores ≥ 5 were selected on the basis of a previous study [11].
2.5 Statistical Analysis
Statistical analysis was performed in STATA 14.0 (Stata Corp LP, city, state, country). The combined OR and 95% CI were calculated, and the Z-test was used to determine whether the combined OR values were statistically significant at p < 0.05. Cochran's Q test, quantified by the I2 statistic [36], was applied to assess the heterogeneity. A random-effects model was used to calculate the combined OR for I2 > 50%. Begg's and Egger's tests were used to detect the publication bias (with p < 0.05 considered statistically significant). Sensitivity analysis was performed with a leave-one-out design to assess the study stability.
3 Results
3.1 Study Selection
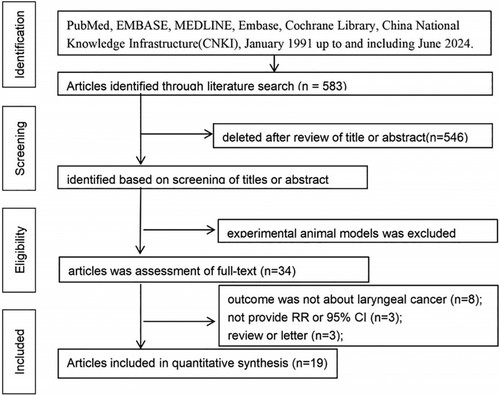
As shown in Figure 1, 583 studies were screened during the initial search, among which 548 did not meet the inclusion criteria for content, study design, target population, and outcomes and were excluded according to the titles and abstracts. The remaining 33 studies were completely and carefully reviewed. The current study included 18 case–control studies and one cohort study. Three studies were from Germany [11, 13, 14]; five were from the US [6, 8, 9, 12, 15]; and one each were from Turkey [3], Estonia [4], France [5], China [7], Uruguay [10], New Zealand [17], Sweden [18], Switzerland [19], and Spain [22]. The 2003 Berrinol study [25] included studies from France, Italy, Spain, and Switzerland. The 2006 Shangina study [20] included studies from Poland, Romania, Russia, and Slovakia (Table 1).
| No. | Author (years) | Study | Country (city) | Follow year | Case | Control | RR/OR | 95% CI | Adjustment for confounding factors | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Elci OC (2002) [12] | Case–control study | Turkey | 1979–1984 | 940 | 1519 | 1.1 | 0.8 | 1.4 | Age, smoking, drinking |
| 2 | Jayaprakash V (2008) [13] | Case–control study | Estonia | 1982–1998 | 124 | 1522 | 1.52 | 0.79 | 2.86 | Age, education, smoking |
| 3 | Laforest L (2000) [14] | Case–control study | France | 1989–1991 | 296 | 296 | 1 | 0.58 | 1.72 | Age, smoking, drinking |
| 4 | Muscat JE (1991) [15] | Case–control study | USA (New York) | 1985–1990 | 194 | 184 | 1.7 | 0.7 | 4.6 | Age, education, drinking |
| 5 | Zheng W (1992) [16] | Case–control study | China | 1989–1990 | 201 | 414 | 1.4 | 0.6 | 3.2 | Age, smoking |
| 6 | Zagraniski RT (1986) [17] | Case–control study | USA (New Haven) | 1975–1980 | 87 | 153 | 2.5 | 0.5 | 12.9 | Smoking, drinking |
| 7 | Brown (1998) [18] | Case–control study | USA (Texas) | 1975–1980 | 183 | 250 | 1.51 | 0.86 | 2.67 | Age, smoking, drinking |
| 8 | De Stefani (1998) [19] | Case–control study | Uruguay | 1993–1995 | 112 | 509 | 1.6 | 0.6 | 4.2 | Age, environment, education, smoking, drinking |
| 9 | Ramroth H (2008) [20] | Case–control study | Germany | 1998–2000 | 257 | 769 | 2.1 | 1.2 | 3.9 | Smoking, drinking, education |
| 10 | Langevin SM (2013) [21] | Case–control study | USA (Boston) | 2006–2011 | 213 | 1442 | 1.2 | 1 | 1.3 | Age, gender, race, smoking, drinking, education, HPV16 |
| 11 | Maier MH (1997) [22] | Case–control study | Germany (Heidelberg) | 1988–1989 | 164 | 656 | 2 | 1.1 | 3.9 | Smoking, drinking |
| 12 | Ahrens W (1991) [23] | Case–control study | Germany (Bremen) | 1986–1987 | 85 | 100 | 0.7 | 0.2 | 2.2 | Age, smoking, drinking |
| 13 | Wortley P (1992) [24] | Case–control study | USA (Washington) | 1983–1987 | 235 | 547 | 0.5 | 0.2 | 1.5 | Age, smoking, drinking, education |
| 14 | Berrino F (2003) [25] | Case–control study | France /Italy/Spain/Switzerland | 1979–1982 | 213 | 819 | 0.6 | 0.3 | 1.3 | Age, area, drinking, income |
| 15 | Kawachi I (1989) [26] | Case–control study | New Zealand | 1980–1984 | 379 | 735 | 0.79 | 0.43 | 1.46 | Age |
| 16 | Purdue MP (2006) [27] | Cohort study | Sweden | 1971–2001 | 227 | 307,799 | 0.8 | 0.50 | 1.5 | Age, smoking |
| 17 | Gustavsson P (1998) [28] | Case–control study | Switzerland | 1988–1990 | 157 | 641 | 0.54 | 0.32 | 0.93 | Age, smoking, drinking |
| 18 | Shangina O (2006) [29] | Case–control study | Poland, Romania, Russia, Slovakia | 1999–2002 | 316 | 728 | 0.81 | 0.48 | 1.37 | Age, country, smoking, alcohol |
| 19 | Pollan (1995) [30] | Case–control study | Spain | 1982–1985 | 43 | 46 | 2.69 | 0.94 | 7.67 | Smoking, drinking |
3.2 Meta-Analysis of Wood Dust Exposure and Laryngeal Cancer
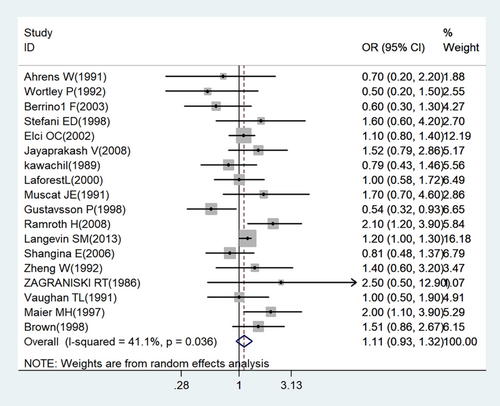
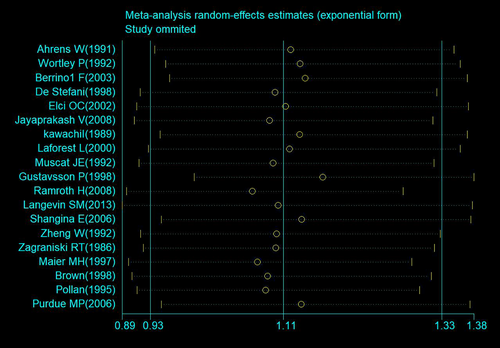
Nineteen studies (18 case–control studies and one cohort study) assessing the association between wood dust exposure and laryngeal cancer were included in the primary meta-analysis (Figure 2). On the basis of the collective study findings, wood dust exposure was associated with elevated laryngeal cancer risk, but the association was not statistically significant (OR: 1.11; 95% CI: 0.931.33 [n = 19]), and moderate heterogeneity was identified (I2 = 45.3%; p = 0.017; Figure 2). Sensitivity analyses indicated no change in the combined OR in the leave-one-out analysis; the OR ranged from 1.07 (95% CI: 0.90–1.28) to 1.17 (95% CI: 0.99–1.38; Figure 3).
3.3 Subgroup Analyses
Wood dust exposure was found to significantly contribute to laryngeal cancer in subgroup analyses based on studies with ≥ 200 cases (OR: 1.14; 95% CI: 1.01–1.25; I2 = 41.1%; p = 0.083 [n = 10]) or < 200 cases (OR: 1.29; 95% CI: 1.00–1.66; I2 = 52.0%; p = 0.03 [n = 9]). A subgroup analysis of wood dust exposure and laryngeal cancer indicated significant associations according to a geographic location in the US (OR: 1.21; 95% CI: 1.07–1.37; I2 = 17.1%; p = 0.306 [n = 5]) or Europe (OR: 1.05; 95% CI: 0.90–1.22; I2 = 53.2%; p = 0.011 [n = 13]).
On the basis of follow-up evaluations, wood dust exposure led to laryngeal cancer after ≥ 5 years (OR: 1.19; 95% CI: 1.07–1.32, I2 = 8.4%; p = 0.365 [n = 10]) or < 5 years (OR: 1.19; 95% CI: 1.07–1.32; I2 = 56.4%; p = 0.014 [n = 9]). Wood dust exposure was associated with laryngeal cancer, on the basis of studies published after the year 2000 (OR: 1.15; 95% CI: 1.04–1.28; I2 = 41.2%; p = 0.104 [n = 8]) or before the year 2000 (OR: 1.12; 95% CI: 0.89–1.42, I2 = 52.5%; p = 0.022 [n = 11]), as shown in Table 2.
| Case | Stduy | OR | 95% CI | I 2 | p |
|---|---|---|---|---|---|
| < 200 | 9 | 1.29 | 1.00–1.66 | 52.0 | 0.034 |
| ≥ 200 | 10 | 1.14 | 1.01–1.25 | 41.1 | 0.083 |
| District | |||||
| European | 13 | 1.05 | 0.90–1.22 | 53.2 | 0.011 |
| USA | 5 | 1.21 | 1.07–1.37 | 17.1 | 0.306 |
| Follow year | |||||
| < 5 | 9 | 0.97 | 0.77–1.21 | 56.4 | 0.014 |
| ≥ 5 | 10 | 1.19 | 1.07–1.32 | 8.4 | 0.365 |
| Published in 2000 | |||||
| Before | 11 | 1.12 | 0.89–1.40 | 52.5 | 0.022 |
| After | 8 | 1.15 | 1.04–1.28 | 41.2 | 0.104 |
3.4 Publication Bias
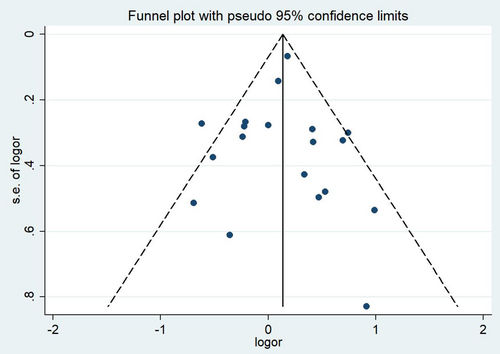
No evidence of publication bias was detected in funnel plots. Moreover, no evidence of study bias was identified with Begg's test (p = 0.675) or Egger's test (p = 0.843), as shown in Figure 4.
4 Discussion
The upper respiratory and digestive tracts are the main pathways through which dust carcinogens enter the human body. Dust can accumulate in the nasal cavity, larynx, and respiratory tract. The location of deposition depends primarily on the dust particle size, shape, and airflow intensity. Dust deposition causes inflammatory responses and acquired respiratory diseases, chronic inflammation, airway obstruction, and chronic obstructive pulmonary disease.
According to the IARC, wood dust is carcinogenic to humans (group 1) and has a definite role in the development of sinonasal cavity tumors [37]. The role of wood dust in the development of cancer has been a subject of substantial research and speculation. A previous study has reported that workers with occupational exposure to wood dust face an elevated risk of nasopharyngeal cancer [38]. Our previous meta-analysis [11] has confirmed that wood dust is associated with nasopharyngeal carcinoma [39]. However, studies exploring the relationship between exposure to wood dust and laryngeal cancer occurrence have yielded inconsistent findings.
The pooled results (OR: 0.95; 95% CI: 0.80–1.17; I2 = 48.8%) of a recent meta-analysis have indicated an association between wood dust exposure and laryngeal cancer risk [22], but the populations in Wortley's et al. 1992 study [24] and Vaughan and Davis 1991 study [32] were from the same case–control study, and the associations investigated in Demers's et al. 1995 study [40], Innos's et al. 2000 study [41], and Laakonen's 2006 study [42] were based on the standardized incidence or standardized mortality ratio. Therefore, the current meta-analysis included only studies reporting results from nonreplicated studies. Laryngeal cancer risk was elevated by wood dust exposure, on the basis of the current meta-analysis (OR: 1.11; 95% CI: 0.93–1.33 [n = 19]), but mild heterogeneity was identified (I2 = 45.3%). To ascertain the sources of heterogeneity, we conducted subgroup analyses on the retrieved studies, which indicated that wood dust exposure increased the risk of laryngeal cancer when a larger number of laryngeal cancer cases were present. The most recent report from the IARC has estimated 184,615 new cases of laryngeal cancer and 99,840 deaths worldwide in 2020 [43]; a larger sample size would make the results more significant and decrease the heterogeneity (I2 = 41.1% < 45.3%). Given the difference in the predominant wood used between Europe and the US, we performed a subgroup analysis by the geographic region. We observed that wood dust exposure was associated with a significantly elevated risk of developing laryngeal cancer in the US, with decreased heterogeneity (I2 = 17.1% < 45.3%). In contrast, among the studies in European countries (particularly Nordic countries), wood dust exposure was not significantly associated with laryngeal cancer. The results by geographic region were consistent with the association between wood dust exposure and lung cancer [44]. Other explanations may exist for the geographic regional differences, including unknown confounding factors and genetic profiles, different occupational and health standards, and levels of health care. Differences in risk estimates might be due to genetic, environmental, or regional differences in health policies. Some European countries are leaders in occupational health and safety practices [45, 46]. To our knowledge, this study reports the first systematic review or meta-analysis assessing wood dust and laryngeal cancer risk.
Case–control studies are effective in identifying and quantifying occupational exposure as a risk factor for cancer. However, the amount of wood dust exposure depends on factors such as the type of wood (hardwood or softwood), wood-processing operations (e.g., sawing, milling, grinding, and sanding), processing (e.g., machine and hand sanding), and wood dryness. Workplace size, ventilation, cleaning, and other workplace features might also affect the levels of wood dust exposure. Herein, we focused on studies assessing wood dust exposure, to provide the most direct assessment of the relationship between wood dust exposure and laryngeal cancer risk. We avoided the effects of wood dust due to occupational classification or type of the wood, in agreement with several studies indicating high variation among individual risk estimates when multiple different occupations associated with wood dust exposure are analyzed in the same study [20, 22]. This further adds to the association between wood dust exposure and laryngeal cancer and suggests that our focus on wood dust-specific studies did not bias the results of this meta-analysis.
Several limitations of this study must be noted. First, wood dust exposure might not have been assessed accurately because the studies used questionnaires to assess wood dust exposure, but self-reporting could easily lead to reporting bias. Second, given the complex etiology of laryngeal cancer, the influence of other possible carcinogens present in the workplace (e.g., solvents, asbestos, or formaldehyde) should be considered in addition to factors such as smoking and age.
5 Conclusions
Despite these limitations, the findings of the current meta-analysis suggest that wood dust exposure in the workplace might increase the risk of laryngeal cancer. This meta-analysis showed an association between wood dust exposure and laryngeal cancer, on the basis of moderate evidence, and a relatively higher risk of laryngeal cancer was observed in studies conducted in the US. Moreover, long-term exposure and larger sample sizes are necessary to provide sufficient evidence to confirm that wood dust exposure is an independent additional risk factor for laryngeal cancer.
Registration and Protocol
The protocol is described in the Section 2. Registration does not apply.
Author Contributions
E. Meng: formal analysis (equal), writing – review and editing (equal). Zhou Xin: methodology (equal), project administration (equal). Dou Jianrui: conceptualization (equal), data curation (equal). Yin Jinzhu: data curation (equal).
Ethics Statement
This article did not involve human or animal research.
Consent
This article did not involve enrollment of human participants.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.