Hyperthermia combined with immune checkpoint inhibitor therapy: Synergistic sensitization and clinical outcomes
Abstract
Background
Within the field of oncotherapy, research interest regarding immunotherapy has risen to the point that it is now seen as a key application. However, inherent disadvantages of immune checkpoint inhibitors (ICIs), such as their low response rates and immune-related adverse events (irAEs), currently restrict their clinical application. Were these disadvantages to be overcome, more patients could derive prolonged benefits from ICIs. At present, many basic experiments and clinical studies using hyperthermia combined with ICI treatment (HIT) have been performed and shown the potential to address the above challenges. Therefore, this review extensively summarizes the knowledge and progress of HIT for analysis and discusses the effect and feasibility.
Methods
In this review, we explored the PubMed and clinicaltrials.gov databases, with regard to the searching terms “immune checkpoint inhibitor, immunotherapy, hyperthermia, ablation, photothermal therapy”.
Results
By reviewing the literature, we analyzed how hyperthermia influences tumor immunology and improves the efficacy of ICI. Hyperthermia can trigger a series of multifactorial molecular cascade reactions between tumors and immunization and can significantly induce cytological modifications within the tumor microenvironment (TME). The pharmacological potency of ICIs can be enhanced greatly through the immunomodulatory amelioration of immunosuppression, and the activation of immunostimulation. Emerging clinical trials outcome regarding HIT have verified and enriched the theoretical foundation of synergistic sensitization.
Conclusion
HIT research is now starting to transition from preclinical studies to clinical investigations. Several HIT sensitization mechanisms have been reflected and demonstrated as significant survival benefits for patients through pioneering clinical trials. Further studies into the theoretical basis and practical standards of HIT, combined with larger-scale clinical studies involving more cancer types, will be necessary for the future
1 INTRODUCTION
Under the influence of social factors such as poor lifestyle, environmental modification, and aging populations, cancer remains a recalcitrant disease that seriously impacts global public health.1 According to the World Cancer Report, in 2020 there were 19.29 million new cancer cases and 9.96 million cancer-related deaths.2 Long-term endeavors have brought limited breakthroughs regarding conventional treatments for cancer recurrences and metastasis.3 Remedial innovations remain the main source of solutions to overcome traditional therapeutic barriers.4
Immunotherapy has developed over the past decades through the mass exploration of tumor immunology; it has become a promising strategy for combating cancer.5, 6 It can now be asserted that immunotherapy has grown from a potential topic of interest into a viable, important application.7 This was demonstrated in 2018, when James P. Allison and Tasuku Honjo were awarded the Nobel Prize in Physiology or Medicine, providing strong evidence that immune checkpoint inhibitor (ICI) treatment has gained mainstream recognition.8 Indeed, the application of anti-PD-L1/PD-1 and anti-CTLA-4 for metastatic melanoma and lung cancer are now included in the National Comprehensive Cancer Network (NCCN) guidelines.9 In the 10 years since the first ICI, Ipilimumab was approved by the FDA for unresectable or metastatic melanoma, numerous clinical studies have demonstrated that ICIs provide substantial survival benefits to patients in some cancer subtypes such as Hodgkin's lymphoma, Merkel cell carcinoma, desmoplastic melanoma, or at the tumors with high PD-L1 expression, MSIH/pMMR, and high TMB molecular phenotypes.10, 11 ICI can exert an excellent 50%–90% efficiency in these cancer types,12 but these conditions are relatively rare. In contrast, the response rate to ICI is only about 15%–25% for cancers covering most population, including most lung cancers, MSIL/MSS/dMMR gastrointestinal cancers, breast cancers, prostate cancers, hepatocellular carcinomas, head and neck cancers, urothelial carcinomas, renal cell carcinomas, etc.13-16 In addition, it should also be noted that the development of acquired resistance to ICI remains unavoidable, which is associated with tumor-mediated immunosuppression, defective antigen delivery, neoantigen depletion, and loss of interferon-γ (IFN-γ) signaling, and upregulation of IC bypass expression.17 Therefore, ICI still has significant limitations at present.
Solving the predicaments encountered during traditional or single therapies could contribute to the development of a comprehensive oncotherapy model.18 Currently, improved combined treatments are being collocated by clinicians through the selection of combinations of imperfect treatments.19 For locally advanced gastrointestinal malignancies that are difficult to surgery directly, so neoadjuvant chemotherapy strategies have been proposed in recent decades, which greatly improve the feasibility of surgery and R0 resection rate, thus significantly prolonging survival.20 For pancreatic cancer with highly prone to recurrence after surgery, the pioneering ESPAC-1 study explored the effects of adjuvant chemotherapy.21 The results showed that 5-fluorouracil combined with calcium leucovorin improved the 5-year OS rate to 21%, compared to only 8% with surgery alone. A Meta-analysis involving 1086 cases of NSCLC with brain metastases showed that radiotherapy combined with TKI significantly improved intracranial PFS and OS compared to TKI alone.22 These studies combine the advantages and settle the disadvantages of different remedies, which encourage cancer comprehensive treatment. Strategies that aid the selection of congenial remedies to cover shortcomings and add advantages for ICIs are urgently required; significant efforts are now being made in this regard.23, 24
Physiologic fever is normally a crucial function that improves body immunity.25 Hyperthermia, which entails being subjected to external heat sources, is a novel oncotherapy that can release steerable thermal energy to modulate intratumoral immunology, and thus potentially cure cancer.26 Through developments in medical physics, the clinical operability, precision, and universality of hyperthermia are all currently increasing.27, 28 With the widespread availability of hyperthermia, the amelioration in cancer immunosuppression has been observed in many basic studies,29, 30 leading to the exploration of the potential for combining hyperthermia with ICI treatment (HIT). Emerging results in this regard have not only verified and enriched the theoretical foundation of HIT sensitization but are also now gradually transitioning the development stage of HIT from preclinical explorations to clinical investigations.31-33
Though some articles have pertinently analyzed the mechanism of HIT by focusing on different cells, immunological mediators, and systemic immune status in mice,9, 34, 35 to date there has been no comprehensive review into the connectivity between basic and clinical research, or between animal and human studies. Here, therefore, studies into HIT mechanisms featuring animal models are reviewed, and the molecular and cellular changes are traced through an analysis of their HIT curative efficacy. In addition, new clinical works with different classifications are addressed, and likely future trends in the development of HIT are discussed. This review aims to provide a helpful guide to follow-up practices regarding HIT.
2 MECHANISM AND RESISTANCE OF ICIs
Negative regulation, led by immune checkpoints (ICs), maintains a balance between sensitive exclusion and damage to innocent cells. This regulation can preserve the appropriate immune state under normal circumstances. However, mutated tumor cells can increase the expression of some proteins, such as the up-regulation of PD-L1 expression, which inhibits the killing effect of cytotoxic T-lymphocyte by promoting its binding with PD-1, thus resulting in “immune escape.”36 Based on the above mechanisms, ICIs aim to relieve part of the immunosuppressive signaling pathway and restore the vigor of immune cells.37 The mechanisms of two ICIs (PD-L1/PD-1 and CTLA-4) are summarized briefly in Figure 1.
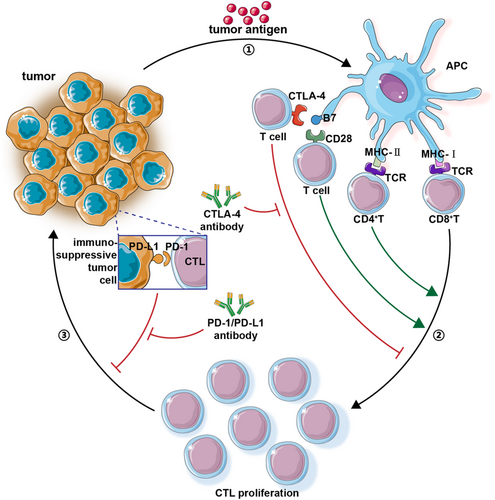
Although ICIs have developed into a promising research direction following rapid development in recent years, overall clinical response rates range from 15% to 60%, suggesting there is a loss in efficacy in practical scenarios compared to theoretical predictions.38 ICIs operate within the tumor microenvironment (TME) under the influence of intratumoral heterogeneity and cellular mutability. This could result in there being an insufficient number of local tumor-infiltrating T cells (TILs), thus decreasing the killing effect of TILs and leading to difficulties regarding the formation of memory T cells. These pathways can inhibit ICIs, leading to a “drug-resistant TME.”39, 40 In 2015, Allison first proposed the concept of immunogenic and non-immunogenic tumors (“hot tumors” and “cold tumors”). The former refers to the presence of high numbers of TILs and interferon-gamma (IFN-γ)+T in the TME, combined with the high expression of PD-L1 in tumor cells; the latter refers to the opposite.41, 42 In addition to the gene mutation of tumor cells, whose TMEs can worsen the phenotype, independent of other directions, mutations can affect ICIs, host immunity, and intestinal microecology. They can also reduce the clinical effectiveness of ICIs.
3 HYPERTHERMIA
Hyperthermia is a kind of physical therapy that uses the biological thermal effect and physical factors of non-ionizing radiation to heat tissue, thus killing tumor tissue or promoting tumor cell apoptosis.43 It can be divided into whole-body hyperthermia and local hyperthermia according to its scope, and into radiofrequency, ultrasound, microwave, infrared, intracavitary perfusion, and nanoparticle hyperthermia according to the heating factor being used.44 Thus, hyperthermia is not only flexible regarding its heating form but also regarding its different curative purposes, thus deriving dual pattern hyperthermia.45
Ultra-hyperthermia (UH) refers to thermal ablation, in which the temperature often rises above 50°C; it is used to kill carcinoma directly. UH includes microwave/radiofrequency ablation, high-intensity focused ultrasound (HIFU), and nanomaterial-based hyperthermia.46 Meanwhile, mild hyperthermia (MH) is conducted at temperatures below 45°C; it is mainly used to assist oncotherapy. MH utilizes the pathophysiology of inordinate vessels and unsound thermoreceptors, which can yield a difference of 3°C between neoplasm and normal tissue.47 Regarding the anti-tumor performance of hyperthermia, UH should mainly be applied to the irreversible denaturation of proteins, whereas induced apoptosis through MH might be more suited to impairing malignancies. The different curative purposes of UH and MH determine the different indications. In short, UH is more suitable for solid tumors with oligometastasis or local progression that are difficult to remove surgically, and some patients can achieve the same results as surgery by UH. For example, thermal ablation for patients with a single lesion ≤5 cm in diameter or for patients with 2–3 lesions and the largest lesion ≤3 cm in diameter in hepatocellular carcinoma can be curative, and the adverse events are significantly lower than those of surgery.48 While MH prefers patients with advanced metastatic cancer, such as intracavity hyperthermia chemotherapy mainly for multiple metastases in the peritoneum, pleura, bladder, and other tissues, especially those who have developed malignant effusions. The membrane permeability of cancer cells can be increased by MH which facilitates the entry of chemotherapeutic drugs to exert cytotoxic effects.49 Moreover, whole-body hyperthermia induces thermogenic factors to systemic thermal stress, which greatly mobilizes the host immunostimulation. This could be a potent adjuvant regimen for comprehensive medication to be suitable for extensive metastasis or patients receiving palliative treatment. In general, the indications of hyperthermia are quite flexible due to the variety of modalities and types of heating, and physicians can choose hyperthermia mode according to the equipment available in their institutions.
Among the multitudinous combination treatments program that includes hyperthermia, it is important to note that HIT is fundamentally different from ICI combined with conventional therapy. Though chemotherapy8 and radiotherapy50 are both also immunostimulatory, the cytotoxicity difference between conventional treatments and hyperthermia regarding normal immunity could lead to the latter having a relatively positive impact on tumor immunology, unlike other radical therapies. Though the limited damage that hyperthermia causes to immunization would not compensate for its initial influence on immunoreactivity, it could help to form a curative immunosensitive TME to aid ICI activation.
4 SYNERGISTIC SENSITIZATION OF HIT
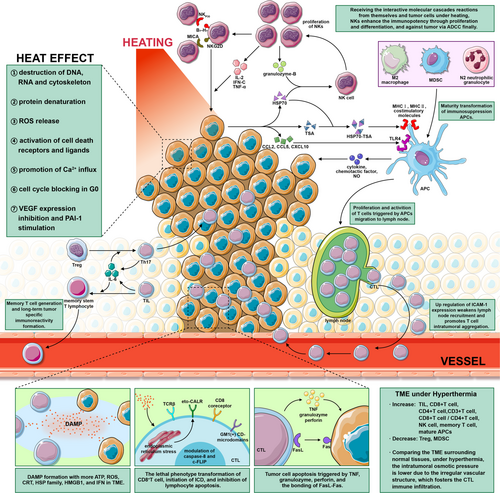
The pharmacologic actions of ICIs can be enhanced by applying thermal stress to the IC axis; this is called direct sensitization. Indirect sensitization, meanwhile, comprises a series of positive immunoregulation paralleling direct mechanisms. The fact that heat can have these effects independent of immunostimulation further reinforces the power of HIT against carcinoma. Several key mechanistic pathways in HIT synergistic sensitization are shown in Figure 2.
4.1 Direct sensitization
Oncologists are currently expanding the theoretical cognitive dimensions of ICs, which is, in turn, creating further opportunities for thermophysical biology to provide new entry points to direct sensitization.51 Hyperthermia can trigger a series of multifactorial molecular cascade reactions between tumors and immunization, thereby significantly inducing cytological modifications within the TME.52 The immunomodulatory amelioration of immunosuppression, combined with the activation of immunostimulation, can greatly enhance the pharmacological potency of ICIs.
Both tumor cells and dendritic cells (DCs) can transform intracellular and extracellular secretion under thermal stimulation. These processes promote the activation and maturity of antigen-presenting cells (APCs), leading to their differentiation into subtypes, accompanied by the modification of T cell phenotypes.53-55 For example, it has been shown that the differentiation of T lymphocytes into Th2 and regulatory T cells (Tregs) in the spleen can be restrained by heating at 39–40°C; the multiplication of Th1 and Tc1 cells increases under these conditions.56 Within the human body, Tregs have also been shown to differentiate into Th17 under IL-6 due to hyperthermia.57
The immunopotency of CD8+T cells can be enhanced by the membrane aggregation of GM1(+) CD-microdomains, T-cell receptor (TCR)β, and CD8 coreceptors, as well as by the secretion of IFN-γ under heating.58 The translocation of AP-1 and NK-κB can also increase the expression of Fas ligand due to up-regulation by heat shock factor-1 (HSF-1), which ultimately offers a potent killing efficiency.59
Immunogenic cell death (ICD) is an influential part of HIT synergism. Based on the generation of tumor neoantigens, calcium reticulin surface exposure (eto-CALR) can be triggered by the production of reactive oxygen species (ROS) and endoplasmic reticulum stress through hyperthermia; this is a prerequisite of ICD.60 Following the secretion of IFNs and adenosine triphosphates (ATPs), the intratumoral immune microenvironment can develop to form damage-associated molecular patterns (DAMPs). This involves the preponderance of calreticulin (CRT), heat shock protein-70 (HSP70), heat shock protein-90 (HSP90), and high mobility group box 1 (HMGB1).61
The formulation of memory stem T cells can also be increased by hyperthermia, which may be attributed to the mass release of IL-6.62, 63 The curative effect analysis described below revealed that long-term tumor suppression through the generation of anamnestic immunity is crucial for controlling recurrence and prolonging survival.
ICI response rates have been shown to be positively correlated with the tumor mutation burden (TMB).64 Hyperthermia can enhance the TMB by damaging deoxyribonucleic acid (DNA) and impelling the release of neoantigens. Thus, it could guide both specific and non-specific immunity to a large extent.65 Rangamuwa et al.66 observed that 80% of patients treated with bronchoscopic thermal vapor ablation exhibited up-regulated expressions of PD-L1. This suggests and possibly validates the ability of hyperthermia to transform “cold tumors” into “hot tumors.” Meanwhile, the quantities of Treg67 and Th1757 have been shown to be noticeably decreased after hyperthermia, which can relieve immunosuppression to some extent. The multiple actions highlighted above could eventually contribute to both neoplastic vulnerability and immunizing aggressivity, thus offering valid ICI pharmacodynamics that can be inferred and observed. It is possible to benefit from these multifactorial mechanisms by enhancing ICI-mediated immunopotency and unburdening immunosuppression. Thus, the promotion of ICI efficacy by hyperthermia may be observable.
4.2 Indirect sensitization
Indirect sensitization is an immunologic supplement for ICIs. Biological modifications in APCs can augment specific immune functions, such as enhancing the immunoreactivity of cytotoxic T lymphocytes (CTLs). Furthermore, phenotypic remolding can regulate tumor nonspecific immunization.68
The mature transformation of immunosuppressive APCs such as M2 macrophages, N2 neutrophilic granulocyte, and myeloid-derived suppressor cells (MDSCs) can arise in hyperthermia above 43°C.69 Natural Killer (NK) cells are currently being studied regarding HIT nonspecific immunization. The proliferation, migration, and cytotoxicity of NK cells can be greatly enhanced by varying their molecular expression, distribution, and secretion via heating.70, 71 Furthermore, substantial endogenous antigens are released under thermal damage, which can cause natural host vaccines to recruit more antibody-dependent cell-mediated cytotoxicity (ADCC) due to the preponderance of NK cells.72
5 PRECLINICAL STUDIES OF HIT
5.1 Ultra-hyperthermia combined with ICI treatment
In recent years, numerous animal experiments have combined ablation with ICI, their results can serve as a link between basic and clinical studies into HIT. Several of the preclinical studies are summarized in Table 1. In the mouse colon cancer CT26 and melanoma B16 cell models, Shi et al.73 found that the proportion of CD8+T/Tregs increased significantly, as did the secretion of IFN-γ and tumor necrosis factor-α (TNF-α) by TILs. The lethality of ablation is much stronger than that of MH, so it can create more endogenous antibodies. Follow-up results showed that tumor volume was significantly controlled in HIT mice, for which the highest survival rate was achieved. The host immune hyper-responsiveness caused by UH could be the main reason for the abovementioned increases in molecules and cells. Han et al.74 carried out HIFU/RFA and anti-CTLA-4 combined with adjuvant experiments in mice to verify the control of metastases after primary lesion ablation. Combined with the findings of Shi et al.73 not only did the amount of Treg decrease, but the number of MDSCs in the TME also appeared to decline. Han et al.74 also proposed that HIFU/RFA increased the uptake of tumor-specific antigen (TSA) by DCs, which is a common result of antigen exposure and DC aggregation induced by hyperthermia. Their data showed that the CTLs of metastatic lesions increased to 17.31%, while the Treg ratio (31.67%) was significantly lower than that in an untreated state (54.05%); the ratio of CD8+T/Tregs increased fivefold. The distant metastasis of the HIT group, compared with a group that underwent ablation alone, subsided significantly, and there were no obvious adverse reactions.
| Reference | Treatment | Tumor model | Microscopic foundation | Curative advantage in vivo |
|---|---|---|---|---|
| [73] |
|
|
|
HIT significantly reduced the tumor size and prolonged survival in mice. |
| [74] |
|
|
|
Mice in the HIT combined with the adjuvant group achieved 100% survival at day 40 and their distant metastasis model disappeared, and mice in this group had the highest survival rate after simulated recurrence. |
| [75] |
|
|
|
The growth of simulated distant metastases was significantly inhibited in the HIT group. The 60-day survival rate of mice in the HIT group was 83%, while 0% in the rest of the groups. |
| [76] |
|
|
|
HIT prevented lung metastases and prolonged survival. |
| [77] |
|
|
|
HIT prevented distant metastases and prolonged survival. |
| [78] |
|
|
|
HIT prevented and inhibited the metastasis by forming long-term immune memory and prolonged survival. |
| [79] |
|
|
|
The growth of primary lesions and distant metastases was significantly inhibited in the HIT group. |
| [82] |
Magnetic hyperthermia Anti-PD-1 (BE0146, Bio X cell) Anti-CTLA4 (BE0164, Bio X cell) Radiation therapy |
|
|
The combination therapy inhibited the growth of primary lesions and distant metastases but did not significantly prolong survival. |
Considerable nanomaterial-based HIT preclinical studies have also been carried out. Pan et al.75 investigated the use of monodisperse superparamagnetic CoFe2O4@MnFe2O4 nanoparticles in mouse breast cancer. These nanoparticles exhibited more efficient, stable, and controllable heat energy under an alternating magnetic field. In the HIT group, the number of CD8+T cells increased significantly and mortality was the lowest. Hu et al.76 verified the HIT efficacy of the mouse CT26 colon tumor model using a copper-doped nanoscale covalent organic polymer. In the absence of obvious adverse reactions, both primary lesions and metastases were inhibited remarkably in HIT group mice. Furthermore, the survival time of this group was more >50 days, which was better than those of other groups. Luo et al.77 carried out HIT by uniting photothermal conversion agents and ICI; they used AA@PN to encapsulate anti-PD-1 peptides in a hollow gold nanoshell for photothermal therapy. Comparing single photothermal or ICI monotherapy, the HIT group achieved the best curative effect and the lowest mortality. Distant metastasis models have also been incubated to investigate whether focal photothermal treatment could ameliorate systemic immunoreactivity against cancer and whether it could work in lesions located elsewhere. The metastasis model was impeded markedly by HIT, which affirmed this hypothesis. This finding was accompanied by increases in CD3+T, CD4+T, and CD8+T cells that were one to three times higher than those of the control group on a cytological level. Molecular modulation through secondary immunostimulation, induced under locoregional heating in the TME, has also been shown to significantly enhance TNF-α, IL-2, and IFN-γ, accompanied by a decline in IL-10.
5.2 Mild hyperthermia combined with ICI treatment
Huang et al.78 used injectable lipid gel to encapsulate a near-infrared (NIR) photothermal agent and anti-PD-L1. They also controllably released ICIs under a transformation temperature of 39.5°C through NIR heating. Tumors in mice in the HIT group shrunk continuously after irradiation and vanished on the tenth day after therapy. Secular trends in malignancy suppression in the HIT group resulted in the highest 60-day survival rate (50%). A series of digital information also cytologically revealed that this group had a higher CD8+T/CD4+T ratio and a higher percentage of mature DC subtypes (CD11c+/CD80+/CD86+). Compared with single anti-PD-L1 and MH groups, the CD8+T count in the HIT group was 1.5 and 1.4 times higher, respectively. These findings correspond with the findings of Shi et al. regarding surgery and HIT.73 Meanwhile, the expressions of CD4+, CD25+, and FoxP3+ on Tregs were significantly lower in the HIT group than in the other groups, as was the number of MDSCs. The above cytological variations provided proof that HIT can relieve intratumoral immune suppression to some extent. Moreover, the abovementioned authors injected carcinoma cells again 30 days after treatment to detect the long-term immunity to metastases. The HIT group was the only group without metastasis, as it benefited from the establishment of a long-term adaptive immune response. Yu et al.79 encapsulated BMS-202 (an ICI acting on PD-L1) in size-adjustable thermo- and fibrotic matrix-sensitive liposomes. They found that desmoplastic stroma and the intratumoral hypoperfusion of pancreatic carcinoma, which impeded routine treatment, were vanquished by mild hyperthermia combined with ICI treatment. Profiting from the ICI fixed-point release via NIR, they demonstrated that eutherapeutic remission occurred in both primary lesions and metastasis.
5.3 HIT combined with other therapies
Radiotherapy, which is the third kind of major traditional therapy for cancer, has been proved to show strong complementarity in the human body when combined with hyperthermia80 or ICI.81 Therefore, the exploration of HIT combined with radiotherapy constitutes a promising direction in the field of integrative therapy. HIT was investigated based on multiple therapeutics, including MH, anti-PD-L1, anti-CTLA-4, and radiotherapy, by Oei et al.82 They found that both primary lesions and metastases were constrained in the united group and that T cell infiltration with CD3+ recruitment was evident. However, the coherence of therapies did not show any statistical advantage regarding curative effectiveness. It may be that the combinations of heat, radiation, drug dosages, and arrangements used resulted in this unsatisfactory result; it may be that determining the correct arrangement is a complex process, suggesting that future research into a conceptual design is crucial.
Although the accession of eradicative radiotherapy did not provide any survival benefit for mice, research into the combination of HIT with immunomodulatory adjuvants had achieved promising effects. Han et al.74 loaded the imiquimod into nanoparticles, with mice in the relevant group achieving a 100% 40-day survival rate (compared to 0% for other groups). The same experiment also compared the differences in memory T cell generation between each group (surgery, HIFU, surgery with adjuvant, and HIFU with adjuvant). The long-term, substantial generation of memory T cells was observed in the HIFU with an adjuvant group. Furthermore, the 80-day survival rate of this group was also the highest (80%), verifying the control of tumor recurrence.
6 FORWARD TO CLINICAL ADMINISTRATION
Pioneering studies are now exploring the clinical applications of HIT. A number of studies have achieved preliminary results that are significant. Thus, it is possible to validate and interpret some of the conclusions and considerations arising from these basic experiments. Ongoing HIT clinical trials are summarized in Table 2. Thermal ablation is still the most widely and maturely used form of hyperthermia in clinical practice, with the majority of these studies focusing on hepatocellular carcinoma (HCC). Regarding completed trials, Lyu et al.83 performed anti-PD-1 (nivolumab + pembrolizumab) combined with thermal ablation in 33 HCC patients who had previously experienced failure during sorafenib therapy. The objective response rate (ORR) increased 2.4 times, and the progression-free survival (PFS) and median overall survival (mOS) reached 5 and 16.9 months, respectively; these values were higher than those of capecitabine chemotherapy alone (4 and 8 months, respectively).84 Duffy et al.85 combined tremelimumab (anti-CTLA-4) with radiofrequency ablation to access the effects of HIT. They achieved a PFS of 6 months in 57.1% of patients and a 12-month PFS in 33.1%; they also obtained a time to progression (TTP) of 7.4 months and an OS of 12.3 months. One round of intrahepatic nidus ablation was also observed to lead to the shrinkage of other lesions after the application of anti-CTLA-4. This effect may have been related to immune sensitization generated by hyperthermia.
| Trial Identifier | Recruitment status | Title | Study nature | Participant numbers and diseases | Therapeutic regimens | Primary endpoints | Latest results | |
|---|---|---|---|---|---|---|---|---|
| 1 | NCT04220944 | Recruiting | Combined Locoregional Treatment With Immunotherapy for Unresectable HCC |
|
|
|
|
Not yet |
| 2 | NCT03939975 | Completed | Anti-PD-1 therapy Combined With Thermal Ablation for Advanced HCC |
|
|
|
|
|
| 3 | NCT03867084 | Recruiting | Safety and Efficacy of Pembrolizumab (MK-3475) Versus Placebo as Adjuvant Therapy in Participants With Hepatocellular Carcinoma (HCC) and Complete Radiological Response After Surgical Resection or Local Ablation (MK-3475-937 / KEYNOTE-937) |
|
|
|
|
Not yet |
| 4 | NCT03864211 | Active, not recruiting | Thermal Ablation Followed by Immunotherapy for HCC |
|
|
|
|
Not yet |
| 5 | NCT03753659 | Recruiting | IMMULAB - Immunotherapy With Pembrolizumab in Combination With Local Ablation in Hepatocellular Carcinoma (HCC) |
|
|
|
|
Not yet |
| 6 | NCT04652440 | Recruiting | RFA Combined With PD-1 in HCC: Phase II Study |
|
|
|
|
Not yet |
| 7 | NCT04150744 | Recruiting | RFA Plus Carrizumab vs Carrizumab Alone for HCC |
|
|
|
|
Not yet |
| 8 |
NCT01853618 |
Completed | Tremelimumab With Chemoembolization or Ablation for Liver Cancer |
|
|
|
|
|
| 9 | NCT03337841 | Unknown | Pembrolizumab as Neoadjuvant Treatment in HCC |
|
|
|
|
Not yet |
| 10 | NCT02821754 | Active, not recruiting | A Pilot Study of Combined Immune Checkpoint Inhibition in Combination With Ablative Therapies in Subjects With Hepatocellular Carcinoma (HCC) or Biliary Tract Carcinomas (BTC) |
|
|
|
|
54 patients have been enrolled. |
| 11 | NCT03101475 | Active, not recruiting | Synergism of Immunomodulation and Tumor Ablation (ILOC) |
|
|
|
|
Not yet |
| 12 | NCT03393858 | Recruiting | Combination of Immunotherapy and Hyperthermia in Advanced Malignant Mesothelioma |
|
|
|
|
Not yet |
| 13 | NCT03757858 | Recruiting | Hyperthermia Combined With Immunotherapy in the Treatment of Abdominal and Pelvic Malignancies or Metastases |
|
|
|
|
Not yet |
| 14 | NCT03959761 | Unknown | Tolerance of Intraperitoneal (IP) Nivolumab After Extensive Debulking Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Patients With Advanced Ovarian Carcinoma (ICONIC) |
|
|
|
|
Not yet |
| 15 | NCT04889768 | Not yet recruiting | HIPEC Combined With Camrelizumab, Paclitaxel, and S-1 for Conversion Therapy in Patients With Advanced Gastric Cancer With Peritoneal Metastasis |
|
|
|
|
Not yet |
| 16 | NCT04156087 | Recruiting | Progression-free Survival After MWA Plus Durvalumab and Tremelimumab for Unresectable Locally Advanced Pancreatic Cancer (MIMIPAC) |
|
|
|
|
Not yet |
| 17 | NCT04805736 | Recruiting | Microwave Ablation Combined With Camrelizumab in the Treatment of Early Breast Cancer |
|
|
|
|
Not yet |
| 18 | NCT03237572 | Recruiting | Focused Ultrasound and Pembrolizumab in Metastatic Breast Cancer (Breast-48) |
|
|
|
|
Not yet |
| 19 | NCT02469701 | Terminated | Advanced Non-Small Cell Lung Cancer Progressing After at Least One Prior Therapy For Metastatic Disease |
|
|
|
|
|
| 20 | NCT04116320 | Recruiting | Focused Ultrasound Ablation and PD-1 Antibody Blockade in Advanced Solid Tumors (AM-003) |
|
|
|
|
Not yet |
Besides HCC, Xie et al.86 applied tremelimumab in HIT to treat biliary tract cancer. Even though 80% of the participants had progressed after second-line chemotherapy, the PFS was still longer than that obtained in a large retrospective analysis of second-line chemotherapy (3.4 months vs. 2.8 months).87 In addition, only 10% of the patients observed grade 3 or 4 irAEs. Kleef et al.88 studied stage IV triple-negative breast cancer patients with lung metastases; these patients received HIT therapy comprising 13.56 Hz of local radiofrequency and IL-2 induced general hyperthermia, combined with immunotherapy (nivolumab+ipilimumab). As a result, the Karnofsky performance score increased from 80 to 100, the pulmonary metastasis was a complete response (CR), and a final survival time of 27 months was reached. Based on this research, Kleef et al.89 also performed a retrospective study of 131 patients with different types of cancer. The immunotherapy plan comprised ipilimumab (0.3 mg·kg−1) plus nivolumab (0.5 mg·kg−1), and cyclophosphamide was used to decrease the number of Tregs. For hyperthermia, based on the above-mentioned radiofrequency and IL-2, water filter infrared whole-body hyperthermia was performed. Finally, an ORR of 31.3% and a PFS of 10 months were attained. Wei et al.90 conducted a non-small cell lung cancer (NSCLC) perspective study involving 21 patients who could not undergo surgery and targeted therapy, to assess the effects of HIT. Camrelizumab (200 mg, q2w) was infused from the 5th to 7th days after microwave ablation until progression. Eventually, an ORR of 33.3% was obtained, two patients achieved CR, five achieved PR, and a mOS of 5.1 months was reached.
Although the above results have inspired HIT to be incorporated into clinical practice, more large-scale clinical trials are needed to better guide the current understanding of the thermal dose, adverse reactions, and applicable population. Matsumoto et al.91 revealed that the maturation of DCs occurred when both DCs and tumors received continuous thermostimulation. This may mainly be attributed to HIT sensitization being affected by intricate multicellular and polymolecular cascades. Chen et al.92 found that sequential hyperthermia could reduce the density of Tregs in TME, but that single hyperthermia could not. Incomplete ablation has also been reported to promote cancer metastasis, but expanding the ablation range will inevitably cause secondary death damage to surrounding normal tissues.93 Therefore, it is crucial to optimize the temperature and scope regarding HIT. Studies have also addressed the adverse reactions of HIT, which should be analyzed through the effect of hyperthermia on irAEs. Regarding the application of anti-CTLA-4, the toxicity of the gastrointestinal tract, skin, and pituitary should be monitored.94 The adverse reactions of the lung, thyroid, and myasthenia gravis should also be scrutinized when using anti-PD-1/PD-L1.95 ICIs have been associated with myocarditis and more severe mortality in multiple ICI treatment groups (0.27%:0.06%, p < 0.001), suggesting that attention should also be paid to this issue regarding HIT.96 Kleef found that only 23.66, 16.03, 6.11, and 2.29% of patients observed irAEs of grades 1, 2, 3, and 4, respectively.89 This indicates that hyperthermia could potentially ameliorate irAEs. This may arise from the lower drug doses that can be used under sensibilization. However, hyperthermia can change the distribution of circulating blood volume by regulating blood vessels. It is not clear whether this will affect the occurrence of ICI-related myocarditis; follow-up research should address this question.
Any therapy transition from the lab toward the ward is gradually step-by-step, and the same is true for HIT as an emerging discipline. As of now, the implementation of hyperthermia is still far less than the demand. The cost-effectiveness of HIT is a considerable factor influencing the psychological acceptance of patients. For UH, several studies have shown that thermal ablation exhibit superior cost-effectiveness compared to surgery.97, 98 Although MH is primarily used as an adjunct to treatment as an expansion option, its addition does not result in lower cost-effectiveness. Trevor D Hamilton's study showed that the addition of HIPEC had better cost-effectiveness than systemic chemotherapy alone for cancer with peritoneal metastases.99 Considering the above views, it can be inferred that the addition of hyperthermia could increase the cost-effectiveness of ICI, which effectively improves psychological acceptance. On the other hand, for patients with a limited understanding of hyperthermia, it is crucial to do well doctor-patient communication to eliminate patients' doubts and to explain and handle AEs properly. At present, there is still potential for further expansion of HIT indications. Under the premise of excluding contraindications such as thermanesthesia, bleeding tendency, unstable vital signs, and severe cardiopulmonary dysfunction, patients receiving ICI are widely administrable candidates for extracorporeal thermogenic MH (e.g., whole-body hyperthermia, local external irradiation). To determine whether patients with pleural or peritoneal metastases can be treated with intracavity hyperthermia chemotherapy based on ICI, the patient's tolerance to chemotherapy should also be taken into account. Meanwhile, patients with the propensity for bleeding and infection should be strictly limited to intracavity hyperthermia chemotherapy due to the invasiveness. Regarding the radicalness of UH, the indication HIT involving UH should focus on the reference of whether patients are suitable for ablation. After clearly meeting ablation indications, the application of ICI should consider the condition and the curative biomarker, such as advanced multiple metastases, and molecular subtypes identified as PD-L1 positive or dMMR/MSIH, which can be systemic administration of ICI after hyperthermia. Overall, it makes sense to enrich the availability of HIT, and it deserves to be more actively incorporated into comprehensive treatment based on the acceptance of patients. Medical institutions should also strengthen training on quality supervision and standardization of hyperthermia, and add hyperthermia equipment to provide more therapeutic options and better hardware bases for physicians and patients.
7 OUTLOOK
Many outcomes from human body studies will be published in the near future; it is expected that these results will provide causes for optimism. Nevertheless, more in vitro studies are needed to reduce the opportunity costs of clinical program selection. The future progression of HIT relies on the development of hyperthermia and ICIs. To exploit potential ICs, it is essential to explore new inhibitors, define ICI biomarkers, improve ICI efficacy, and reduce the side effects; these efforts will help to realize this promising avenue for future cancer immunology.100 For hyperthermia, meanwhile, major directions for future research will include real-time thermometry, precise thermal control, and exact heating.101 Magnetic resonance (MR), computed tomography (CT), and ultrasonography (US)-based three-dimensional (3D) non-invasive thermometry techniques are developing rapidly.102 The advanced infrared real-time thermometry instrument can also conduct two-dimensional (2D) thermography via energy distribution detection.103 The above technologies provide a better platform for quality control regarding hyperthermia.
By virtue of their special physicochemical properties, nanomaterials can exert many superior effects. They may gradually lead to a breakthrough for precise hyperthermia in the future. Nanomaterial-based drug delivery platforms are deemed to represent a new model of “targeted therapy,” which can optimize pharmacokinetics, improve bioavailability, and reduce toxicity. DAMP triggered by hyperthermia offers a recognizable targeting platform for nanoparticles with encapsulated drugs. This could become a breakthrough point for exact HIT as a form of next-generation target therapy. Optimizing conventional pharmacokinetics could augment bioavailability and abate the toxicity of ICIs. By utilizing the up-regulated expression of HSP70 protein under heating, a precise photothermal remedy platform has been developed and a eutherapeutic outcome has been achieved in mice.104 Mirroring this, there are plans to further develop nano-targeted photothermal therapy while loading ICI. To date, nanomedicine has been extensively utilized in HIT, such as in ICI-encapsulated controlled-release patches, thermosensitive drug-carrying gel, photothermal sensitizers, and nano-adjuvants.74, 105, 106 These applications have shown exciting results and promise an exciting future regarding nano-oncology.
A heterojunction WO2.9-WSe2-PEG nanoradiosensitizer constructed by Dong et al.107 has realized in vivo radiotherapy, hyperthermia, and immunotherapy. This achievement can build a bridge for HIT-based combination therapy. These practices will aid the exploration of wider combination remedies, such as targeting, surgery, and chemotherapy on multiple levels.
8 CONCLUSIONS
Based on the limitations of monotherapy regarding advanced cancer, and building on the verification of the multifactorial influences of hyperthermia on tumor immunization, it is necessary to continue to explore HIT, and to promote deeper and broader investigations. Several HIT sensitization mechanisms have not only been observed and explained in numerous basic experiments but have also been reflected and demonstrated as significant survival benefits for patients through pioneering clinical trials. Further studies into the theoretical basis and practical standards of HIT, combined with larger-scale clinical studies involving more cancer types, will be necessary for the future.
AUTHOR CONTRIBUTION
Pengyuan Liu, Mengna Ye, and Yajun Wu contributed equally to this review and should be considered the co-first author. Conceptualizations, Pengyuan Liu and Zhibing Wu; literature collecting and preparation, Mengna Ye and Yajun Wu; writing-original draft preparation, Pengyuan Liu; writing-review and editing, Pengyuan Liu, Mengna Ye, Yajun Wu, Lichao Wu, and Kaiping Lan; Supervision, Zhibing Wu, Kaiping Lan.
ACKNOWLEDGMENT
This work was funded by the National Natural Science Foundation of China (82172679), Zhejiang Natural Science Foundation (LGF18H160037), Zhejiang Provincial Medicine and Health Science Foundation (2021KY010), Hangzhou Science Foundation (20171226Y223), and Zhejiang Hospital Level Research Project (2020KYA018).
CONFLICT OF INTEREST
The authors declare that they have no competing interests. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution, and reproduction in any medium provided the original work is properly cited.
ETHICS APPROVAL
This study was approved by the Ethics Department of Zhejiang Hospital.
Open Research
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.




