Contribution of MLH1 constitutional methylation for Lynch syndrome diagnosis in patients with tumor MLH1 downregulation
Abstract
Constitutional epimutation of the two major mismatch repair genes, MLH1 and MSH2, has been identified as an alternative mechanism that predisposes to the development of Lynch syndrome. In the present work, we aimed to investigate the prevalence of MLH1 constitutional methylation in colorectal cancer (CRC) patients with abnormal expression of the MLH1 protein in their tumors. In a series of 38 patients who met clinical criteria for Lynch syndrome genetic testing, with loss of MLH1 expression in the tumor and with no germline mutations in the MLH1 gene (35/38) or with tumors presenting the BRAF p.Val600Glu mutation (3/38), we screened for constitutional methylation of the MLH1 gene promoter using methylation-specific multiplex ligation-dependent probe amplification (MS-MLPA) in various biological samples. We found four (4/38; 10.5%) patients with constitutional methylation in the MLH1 gene promoter. RNA studies demonstrated decreased MLH1 expression in the cases with constitutional methylation when compared with controls. We could infer the mosaic nature of MLH1 constitutional hypermethylation in tissues originated from different embryonic germ layers, and in one family we could show that it occurred de novo. We conclude that constitutional MLH1 methylation occurs in a significant proportion of patients who have loss of MLH1 protein expression in their tumors and no MLH1 pathogenic germline mutation. Furthermore, we provide evidence that MLH1 constitutional hypermethylation is the molecular mechanism behind about 3% of Lynch syndrome families diagnosed in our institution, especially in patients with early onset or multiple primary tumors without significant family history.
Introduction
Lynch syndrome is the most common hereditary syndrome that predisposes to colorectal cancer (CRC), corresponding to 2-5% of all CRCs 1, 2. It is an autosomal dominant disease caused by germline mutations in the Mismatch Repair (MMR) genes involved in DNA repair 3, 4. These include MLH1, MSH2, MSH6, and PMS2, although about 90% of the mutations described in this syndrome occur in MLH1 or MSH2 5-8. In the past decade, another distinct mechanism affecting the two key MMR genes MLH1 and MSH2 was unraveled in a subset of patients meeting the clinical criteria for LS without a germline MMR mutation, termed as “constitutional epimutations” or just “epimutation” 9, 10. This phenomenon consists in constitutional transcriptional silencing of these genes by epigenetic mechanisms rather than by genetic mutations that directly affect the sequence of the gene 8-10.
Constitutional epimutations are stable epigenetic abnormalities that are present in normal tissues 11. Constitutional epimutations of the MSH2 gene are secondary to germline deletions in the EPCAM gene in cis, being transmitted in an autosomal dominant fashion just like germline MMR mutations 12. However, constitutional epimutations of the MLH1 gene are more variable, and the pattern of transmission of these distinct forms of MLH1 epimutation presumably reflects their mechanistic basis. MLH1 epimutations may be dichotomized into two categories: (1) those that arise spontaneously and are reversible between generations, though occasionally transmitted to the next generation in a non-Mendelian pattern (primary MLH1 epimutation); and (2) Mendelian epimutations that follow a classic autosomal dominant inheritance pattern due to an underlying cis-genetic cause (secondary/genetically facilitated MLH1 epimutation) 10, 13.
Few cases of constitutional MLH1 methylation have so far been reported 10-13. Furthermore, the exact prevalence of MLH1 constitutional epimutations is still unknown, as most studies addressing this issue were based on series enriched for patients with CRC diagnosed at an age of onset below 50 years 8, 14-16.
Materials and Methods
Patients, samples, and DNA extraction
The study includes a consecutive series of peripheral blood lymphocyte (PBL) samples from 38 patients (index cases, 17 males and 21 females), who meet clinical criteria for Lynch syndrome genetic testing and had loss of MLH1 expression in the tumor (relevant clinical information in Table 1). Three of these patients (3/38) presented the p.Val600Glu BRAF somatic mutation (and therefore were presumed not to carry a germline mutation), whereas the remaining 35 had a negative genetic test for MLH1 deleterious germline mutations. These patients were diagnosed and surgically treated at the Portuguese Oncology Institute of Porto, assessed through genetic counseling, and referred to the Genetics Department of this institution between 2009 and 2014. The majority of these patients (37/38; 97.4%) met the Bethesda criteria and one family (1/38; 2.6%) met the Amsterdam criteria. Clinicopathological information was obtained from medical records.
| Patient | Gender | Tumor localization (diagnosis age) | IHC MMR | Clinical criteria | BRAF | MLH1 |
|---|---|---|---|---|---|---|
| #1 | F | Ascending colon (40) | MLH1/PMS2 absence | BC | NA | WT |
| #2 | F | Stomach (75)Cecumc (75)Breast (78) | MLH1 absencea | BC | NA | WT |
| #3 | M | Descending colon (38 and 48) | MLH1 absencea | BC | WT | WT |
| #4 | M | Ascending colon (25) | MLH1/PMS2 absence | BC | WT | WT |
| #5 | M | Sigmoid colon (51) | MLH1/PMS2 absence | BC | WT | WT |
| #6 | M | Rectum (53) | MLH1/PMS2 with decreased immunoreactivity | BC | WT | WT |
| #7 | M | Ascending colon (43) | MLH1/PMS2 absence | BC | WT | WT |
| #8 | F | Rectum (16) | MLH1 absence (normal PMS2) | BC | WT | WT |
| #9 | F | Rectum (43) | MLH1 absencea | BC | V600E | NA |
| #10 | F | Ascending colonc (26)Stomach (60) | MLH1/PMS2 absence | BC | WT | WT |
| #11 | M | Ascending colon (65) | MLH1/PMS2 absence | BC | WT | WT |
| #12 | F | Ascending colon (62) | MLH1/PMS2 absence | BC | WT | WT |
| #13 | M | Sigmoid colon (44) | MLH1/PMS2 absence | BC | WT | WT |
| #14 | F | Ascending colon (69) | MLH1/PMS2 absence | BC | WT | WT |
| #15 | F | Uterus and ovary (38) | MLH1/PMS2 absence | BC | WT | WT |
| #16 | F | Breast (60)Ascending colonc (66) | MLH1/PMS2 absence | BC | WT | WT |
| #17 | M | Ascending colon (25) | MLH1/PMS2 absence | BC | WT | WT |
| #18 | M | Sigmoid colon (43) | MLH1/PMS2 absence | BC | NA | WT |
| #19 | M | Sigmoid colon (47) | MLH1/PMS2 absence | BC | WT | WT |
| #20 | M | Ascending colon (23) | MLH1/PMS2 absence | BC | WT | WT |
| #21 | F | Endometrium (57)Ascending Colonc (74)Lung (74) | MLH1/PMS2 absence | BC | V600E | NA |
| #22 | F | Sigmoid colon (47) | MLH1/PMS2 absence | BC | WT | WT |
| #23 | F | Ascending colon (59) | MLH1/PMS2 absence | BC | WT | WT |
| #24 | M | Rectum (45)Ascending colonc (61) | MLH1/PMS2 absence | BC | WT | WT |
| #25 | F | Ascending colon (62)Endometriumc (63) | MLH1/PMS2 absence | BC | WT | WT |
| #26 | F | Ascending colon (41) | MLH1/PMS2 absence | BC | WT | WT |
| #27 | M | Rectum (33) | MLH1/PMS2 absence | BC | WT | WT |
| #28 | F | Stomach (78) | MLH1/PMS2 absence | BC | WT | WT |
| #29 | F | Breast (30) | MLH1/PMS2 absenceb | AC | WT | WT |
| #30 | M | Sigmoid colon (61) | MLH1/PMS2 absence | BC | WT | WT |
| #31 | F | Descending colon (65) | MLH1/PMS2 absence | BC | V600E | NA |
| #32 | F | Ascending colon (54) | MLH1/PMS2 absence | BC | WT | WT |
| #33 | F | Ascending colon (42) | MLH1/PMS2 absence | BC | NA | WT |
| #34 | M | Ascending colon (60) | MLH1/PMS2 absence | BC | WT | WT |
| #35 | M | Ascending colon (44) | MLH1/PMS2 absence | BC | NA | WT |
| #36 | F | Endometrium (50) | MLH1/PMS2 absence | BC | WT | WT |
| #37 | F | Sigmoid colon (56) | MLH1/PMS2 with decreased immunoreactivity | BC | WT | WT |
| #38 | M | Ascending colonc (48)Transverse colon (48)Descending colon (48) | MLH1/PMS2 absence | BC | WT | WT |
- AC, Amsterdam criteria; BC, Bethesda criteria; F, female; IHC, immunohistochemistry; M, male; MMR, mismatch repair; NA, not analyzed; WT, wild-type.
- a The analysis was not performed for PMS2.
- b IHC was performed on tumor of a relative.
- c Tumor used for IHC MMR (when cases presented multiple tumors).
Whenever possible, family members of the index patients were also studied, along with buccal swab samples and paraffin-embedded tissue samples (with different germ layer origins) from patients harboring constitutional epimutation. DNA from PBL samples was obtained using the Magna Pure LC 2.0 (Roche Applied Science, Indianapolis, Indiana) and RNA extraction from PBL was performed using Trizol reagent (Invitrogen) according to the manufacturer's instructions and standard protocol 17. The DNA in paraffin histological sections was isolated with the QIAamp® DNA FFPE Tissue Kit (Qiagen, Hilden, Germany) following the manufacturer's instructions. The buccal mucosa was collected with a swab and preserved in a dry environment and DNA was extracted according to standard procedures.
Methylation analysis by MS-MLPA
Analysis of MLH1 promoter methylation was performed on PBL DNA of the index patients. Methylation testing was performed by Methylation-Specific Multiplex Ligation-Dependent Probe Amplification (MS-MLPA) using the SALSA MS-MLPA ME011-B1 kit (MRC Holland, Amsterdam, Netherlands). Whenever possible, buccal swab samples and paraffin-embedded tissues were analyzed for MLH1 promoter methylation using the same approach. The six probe pairs in the MLH1 promoter (with the respective ligation sites located at −659, −518, −382, −246, −13, and +206 relative to the start codon, LRG_216t1) cover independent regions: regions A to D of the promoter and intron 1. The most important methylation region associated with MLH1 silencing is the C-Deng region, from −248 to −178nt before the transcription site, and the second most important region is the D-Deng region, from −9 to +15 nt 14, 18. Fragments were analyzed using the GeneMapper software (Applied Biosystems, Foster City, CA, USA). DNA Quantity (Q-fragments) and DNA Denaturation (D-fragments) control fragments present in the MS-MLPA probe mix were checked. Aberrant methylation was assessed by comparison with normal reference samples. Two intraexperimental replicates in two independent experiments were analyzed and the mean was calculated. In all cases with MLH1 promoter constitutional methylation an independent DNA PBL extraction was performed and subsequently analyzed by MS-MLPA.
Additionally, the SALSA MLPA probemix ME042-C1 CIMP kit (MRC Holland) was used as described above in PBL samples. This probemix enables detection of aberrant methylation of CpG islands around the transcription start site of eight genes (CACNA1G, CDKN2A, CRABP1, IGF2, MLH1, NEUROG1, RUNX3, and SOCS1), for which an altered methylation status, related to the CpG Island Methylator Phenotype (CIMP), has been reported in CRC 19, 20.
Bisulfite treatment and DNA methylation analyses by qMSP and ddPCR
DNA from PBL samples (patient #3, #10, #27, #38, and parents of patient #27) was available for bisulfite treatment. The EpiTect Bisulfite kit (Qiagen) was used for the conversion, following the manufacturer’s protocol. The samples were purified using the QIAcube (Qiagen).
Quantitative methylation-specific PCR (qMSP) was used to analyze the C-region of the MLH1 promoter (assay location: −270−194 relative to the transcription start site; forward primer: GCGGATAGCGATTTTTAACGC, reverse primer: CTTCGTCCCTCCCTAAAACGA, probe: 6FAM-AGCGTATATTTTTTTAGGTAGCG-MGB) 21. In addition, a panel of six biomarkers (previously shown to be hypermethylated in 65–94% of tumor samples from colorectal cancer patients 22) comprising CNRIP1, FBN1, INA, MAL, SNCA, and SPG20 was analyzed, using the same primer and probe sequences as previously described 22, 23. The ALU-C4 element 24 was used as a control to normalize for DNA input. A fivefold dilution standard curve (32.5–0.052 ng) was generated from in vitro methylated DNA (IVD; Zymo Research, Irvine, CA) and added in triplicate to 384 well plates together with 3 × 32.5 ng bisulfite-treated DNA template, 3× water (negative control), 3 × 32.5 ng bisulfite-treated DNA isolated from the whole blood of healthy individuals (methylation negative control), and two 3 × 32.5 ng bisulfite converted IVD (methylation-positive controls). 1 x TaqMan Universal PCR Mastermix No AmpErase UNG (Life Technologies), 0.9 μmol/L of each primer, and 0.2 μmol/L probe was added to each well to a total reaction volume of 20 μL. The PCR reactions (95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 60 sec) were carried out in a 7900HT Real-Time PCR System (Life Technologies, Carlsbad, CA).
Samples amplified after cycle 35 were censored according to the recommendations from Life Technologies. To normalize for DNA input, the median quantity of the GENE was divided by the median quantity of ALU 24. The percent of methylated reference (PMR) values were calculated by dividing the normalized quantity of the GENE by the averaged normalized quantity of the two positive control controls, and multiply by 100.
Droplet digital PCR (ddPCR) of the MLH1 promoter was performed on bisulfite-treated PBL samples from the parents of patient #27, using the same MLH1 assay as for qMSP, with a FAM-labeled probe. In addition, a 4plex control assay, marked with VIC, was included to ensure that enough DNA was present for amplification. The ddPCR reactions were performed using the QX200™ droplet digital PCR platform (BioRad, Hercules, CA) following the manufactures’ protocol. Droplets were generated by the QX200 Droplet Generator. Following PCR amplification, the fluorescence signals were measured by the QX200 Droplet Reader and analyzed by QuantaSoft version 1.7.4.0917 (BioRad).
MLH1 promoter sequencing
Screening for mutations within the MLH1 promoter (which may affect the binding of MLPA probes) was performed by Sanger sequencing in the 38 peripheral blood samples and using two sets of primers, according to Pineda et al. 25.
MLH1 transcript quantification in PBL samples
The MLH1 transcripts were quantified by semiquantitative multiplex RT-PCR. For this purpose, we simultaneously amplified a control transcript, the β2-microglobulin (B2M), and part of the MLH1 transcript, using fluorescence-labeled primers and according to the QIAGEN OneStep RT-PCR Kit (Qiagen). MLH1 transcript levels were calculated by comparing the relative peak areas of the MLH1 transcript to the relative peak areas of the B2M. Three independent experiments were performed.
MLH1 allelic expression analysis in PBL samples
In all patients with constitutional methylation and heterozygous for the coding MLH1 polymorphism c.655A>G (rs1799977) in exon 8, allelic expression analyses were determined in genomic DNA and in cDNA by single-nucleotide primer extension (SNuPE) using the SNaPshot Kit (Applied Biosystems) following the manufacturer′s protocol. The results were independently scored by two observers, and a third round of analyses confirmed the results.
Analysis of p.Val600Glu BRAF mutation
The BRAF c.1799T>A, p.Val600Glu (also known as V600E), mutation was screened in the tumors of all 38 patients by PCR amplification and High Resolution Melting (HRM) analysis on a LightCycler-480 II Real-Time System (Roche Applied Science, Indianapolis, Indiana). As a confirmation of this technique SNuPE was performed following the SNaPshot Kit (Applied Biosystems) manufacturer's protocol.
Study of microsatellite instability
Microsatellite instability was performed using the Bethesda panel (BAT25, BAT26, D2S123, D5S346, and D17S250), according to the 1997 National Cancer Institute Guidelines using fluorescence-labeled primers 26. Fragments were analyzed for length variations on an ABI PRISM™ 310 Genetic Analyzer DNA sequencer (Applied Biosystems) and allele sizes were determined using the GeneMapper software. The results were independently scored by two observers and an additional round of analyses confirmed the results.
Results
Identification of patients harboring constitutional MLH1 promoter methylation
Constitutional methylation of the MLH1 promoter was detected in four (4/38; 10.5%) patients (Table 2 and 3; Table S1; Fig. 1), namely cases #3, #10, #27, and #38. The mean age at diagnosis of these patients was 36 years (range 26–48). The methylation level detected in PBL at the C-Deng region (the region most directly involved in MLH1 transcriptional activity) by MS-MLPA was 14.1%, 18.3%, 32.6%, and 46.3% for patient #3, #10, #27, and #38, respectively, indicating that this alteration, at least for some of these patients, might be present in mosaic. MLH1 promoter methylation was also detected by qMSP analyses in the PBL samples from the four probands (Table 3).
| % MLH1 methylation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PBL | Tumor | Normal colon mucosa | Buccal mucosa | Muscle | ||||||
| Patient | C region (-246 nt) | D region (−13 nt) | C region (−246 nt) | D region (−13 nt) | C region (−246 nt) | D region (−13 nt) | C region (−246 nt) | D region (−13 nt) | C region (−246 nt) | D region (−13 nt) |
| #3 | 14.1 | 43.3 | 20.4 | 62.9 | 8.35 | 27.7 | 12.4 | 44.5 | 11.1 | 32.3 |
| #10 | 18.3 | 49.9 | 11 | 32.2 | 9.1 | 30.5 | NAa | NAa | NAa | NAa |
| #27 | 32.6 | 51.4 | 13 | 38.6 | 14.4 | 35.4 | 13.8 | 40.5 | 12.2 | 39.5 |
| #38 | 46.3 | 52.3 | 16.4 | 40.2 | 11.5 | 36 | 11.6 | 36.4 | NA | NA |
- NA, not available; PBL, peripheral blood lymphocytes.
- a Patient died during the study, so it was not possible to study buccal mucosa.
| % Methylation in MLH1 C-region (gDNA) | % MLH1 transcript levels decrease (cDNA) | % decrease A/G allele (cDNA/gDNA) | ||
|---|---|---|---|---|
| Patients | MS-MLPA | qMSP | RT-PCR | SNuPe |
| #3 | 14.1 | 35.4 | 38.0 | NA |
| #10 | 18.3 | 37.0 | 37.4 | 52.5 |
| #27 | 32.6 | 38.2 | 46.2 | 31.6 |
| #38 | 46.3 | 41.9 | 40.7 | NA |
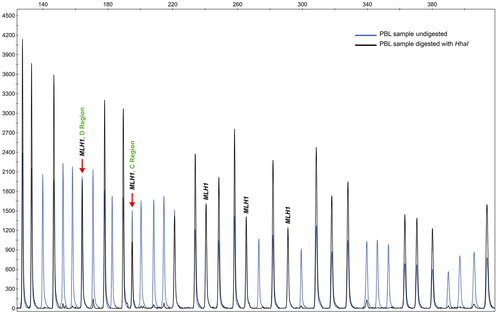
Additionally to PBL samples (mesoderm), we also studied MLH1 promoter methylation in samples representative of all embryonic layers, namely: tumor and normal colon mucosa (endoderm), buccal mucosa (ectoderm), and muscle (mesoderm). MLH1 methylation was present in all tissues analyzed (Table 2), demonstrating that this epigenetic alteration affects tissues from different embryonic origins. On the other hand, analysis of the constitutional methylation status using the MS-MLPA CIMP kit in the four patients with MLH1 epimutation showed that none of the other seven genes tested (CACNA1G, CDKN2A, CRABP1, IGF2, NEUROG1, RUNX3, and SOCS1) presented hypermethylation (Table S2). Additionally, the promoter regions of a panel frequently methylated in CRC 22 (CNRIP1, FBN1, INA, MAL, SNCA, and SPG20) were also unmethylated in the PBL samples from all four probands (Table S3).
We were able to study three relatives (parents and sister) of one of the probands with constitutional methylation of the MLH1 promoter (patient #27), and none of them presented constitutional methylation of the MLH1 promoter by MS-MLPA. The absence of constitutional methylation in the parents was confirmed by ddPCR.
Clinicopathological features of patients with MLH1 constitutional methylation
Case #3 was a male who had two tumors of the descending colon, one at 38 and another at 45 years of age (primary metachronous tumors). The patient had no family history of cancer as shown in the family pedigree (Fig. 2A).
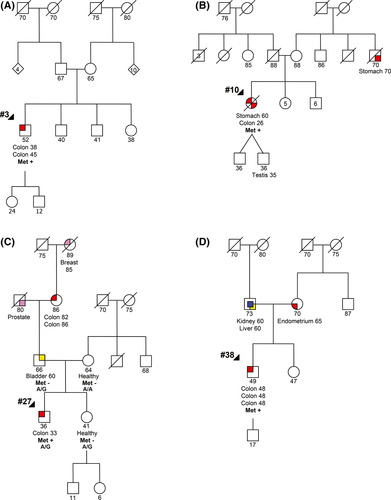
Case #10 was a female who was diagnosed with a moderately differentiated adenocarcinoma in the ascending colon at the age of 26 years and a stomach adenocarcinoma at the age of 60 years, the latter being her cause of death. This patient presented scant family history of cancer, namely a maternal uncle affected with gastric cancer at the age of 70 years (Fig. 2B).
Case #27 was a male who was diagnosed with an invasive adenocarcinoma in the rectum at the age of 33 years. This patient has family history of cancer, namely, a paternal great-grandmother with breast cancer, a paternal grandfather with prostate cancer, a paternal grandmother with colon cancer, and a father with bladder cancer. The father and the healthy mother and sister also participated in this study (Fig. 2C).
Case #38 was a male who was diagnosed with three synchronous adenocarcinomas in the ascending, transverse, and descending colon at age of 48 years. The patient′s father was affected by kidney and liver cancer at the age of 60 years and the mother was affected with an endometrial cancer at the age of 65 years (Fig. 2D).
Microsatellite instability and BRAF analyses
Microsatellite instability analysis was performed by fragment analysis in tumors and normal colon mucosa of all four patients positive for constitutional methylation. The tumors of all cases with constitutional methylation of the MLH1 promoter were MSI-H, with cases #3, #10, and #27 showing instability in 50% of the markers and case #38 in 100% of the markers (data not shown). On the other hand, none of the tumors of the four patients with constitutional methylation of the MLH1 promoter presented the p.Val600Glu BRAF mutation.
MLH1 promoter sequencing
Sanger sequencing of the whole MLH1 promoter (from c.−1469 to intron 1) was performed in PBL samples from all cases, probands (n = 38) and relatives (n = 3), in order to find out if there were any variants which might inhibit the binding of the MS-MLPA probes and to identify promoter variants that might be associated with MLH1 methylation. The results showed that 36 cases did not present variants affecting the binding sites of the MS-MLPA probes or the HhaI restriction sites. Nonetheless, one patient, who did not present constitutional methylation, had the alteration MLH1 c.-261G>A (rs587782685) described as a variant of unknown significance (VUS) in the ClinVar NCBI database (www.ncbi.nih.gov/clinvar). Another heterozygous alteration, the SNP MLH1 c.-269C>G (rs35032294), was found in patient #38. Although these variants occur within a MS-MLPA probe, the fact that no copy number changes were found indicates that they most probably did not affect the hybridization. Finally, the common SNP MLH1 c.-93G>A (rs1800734) was found in heterozygosity in 16 cases (16/38; 42%), including patient #3 with constitutional methylation, and in homozygosity in one case (1/38; 2.6%). This common polymorphism is outside the probe hybridization sites. No other variants were found in the MLH1 promoter region.
Quantification of the MLH1 transcript
To assess global MLH1 transcript levels, we measured its relative expression levels by semiquantitative multiplex RT-PCR in PBL samples of patients with constitutional methylation (#3, #10, #27, #38) and controls. We amplified the MLH1 transcript and the B2M as internal control. As shown by the electrophoretic profile of the multiplex RT-PCR products (Fig. 3A), we observed a decrease in MLH1 RNA expression in the cases positive for constitutional methylation compared to controls.
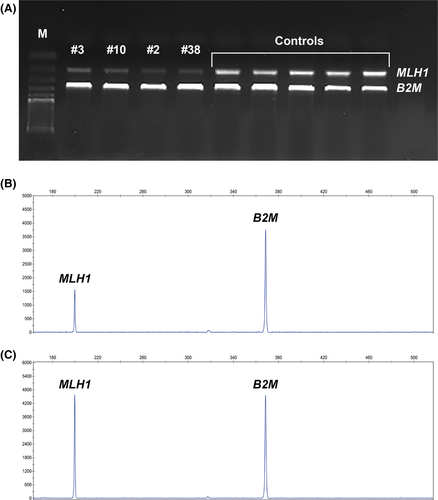
To confirm and measure the differences in the transcript levels observed in the electrophoresis of the multiplex RT-PCR products from patients with constitutional methylation and controls, we performed fragment analysis. Controls presented an average of 99% of MLH1 transcript expression relatively to B2M. Considering the four cases with constitutional methylation, an average decrease in MLH1 transcript levels of 38%, 37%, 46%, and 41% (when compared to B2M) was seen for patients #3, #10, #27, and #38, respectively (Fig. 3B, and C).
MLH1 allelic expression
Data available from the MLH1 germline mutation screening showed that two of the probands positive for constitutional methylation of the MLH1 promoter (patient #10 and #27) were heterozygous for the coding polymorphism c.655A>G, p.(Ile219Val) (rs1799977) in exon 8. The parents and sister of patient #27 were also studied for this polymorphism, the mother being homozygous for the A allele, and the father and sister heterozygous (Fig. 2C). In order to evaluate if the MLH1 promoter methylation was monoallelic, the cDNAs of patients #10 and #27 were sequenced in PBL samples. In both cases, both alleles were present, but one of them appeared to be less expressed (Fig. 4A). This difference was more evident when compared with controls (Fig. 4B).
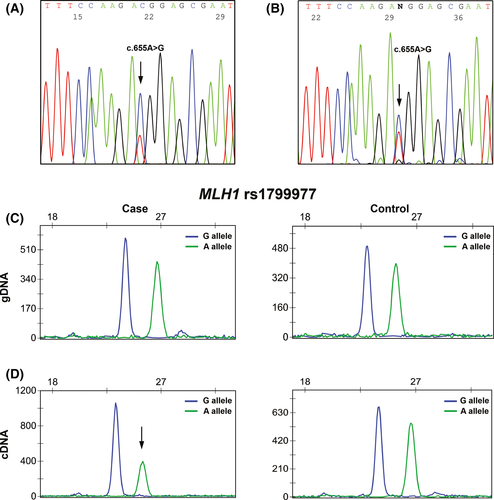
In order to confirm these results, we performed on gDNA and cDNA SNuPe analysis specific for the rs1799977 (c.655A>G), in the two constitutional methylation-positive patients heterozygous for this polymorphism (cases #10 and #27) and in controls (also heterozygous). In the gDNA, no significant differences were obtained in the A/G allele relative areas (case #10: 86%; case #27: 86%; and controls: 88%, Fig. 4C). In the cDNA, we observed that both alleles were expressed in the two probands, but in both cases the A allele showed a signal reduction not observed in the controls (Fig. 4D), suggesting a decrease in the expression of the A allele. We observed a 45% decrease in the A allele relative to the G allele in patient #10 and a 27% decrease in patient #27, whereas in controls the difference between the two alleles was 2%. Moreover, the normalized ratios between cDNA and gDNA revealed a 53% and a 32% decrease in A allele in case #10 and in case #27, respectively, whereas in controls the normalized ratio difference was <0.1%.
Discussion
In this study we identified four patients with constitutional methylation of MLH1. The frequency of constitutional MLH1 methylation was 10.5% (4/38), signifying that MLH1 methylation may account for a non-negligible proportion of Lynch syndrome patients when analysis is restricted to those showing MLH1 abnormal expression in their tumors. In previous reports, the constitutional MLH1 methylation frequency varied between 1.5 and 6% 9, 13, 19, 25, 27-29. Moreover, we show here that constitutional methylation appears to be specific of the MLH1 gene in all four patients with epimutation. In fact, none of the other genes tested by MS-MLPA and qMSP showed constitutional methylation, thus arguing against a generalized disruption of germline methylation patterns in these Lynch syndrome patients. Considering that we had identified 126 families with germline mutations in MMR genes in our institution at the time of this study, MLH1 constitutional hypermethylation seems to be the molecular mechanism behind about 3% of Lynch syndrome families with molecular diagnosis at our institution.
Two of the four patients with constitutional MLH1 methylation identified in this study had developed multiple Lynch syndrome-associated tumors at an early age, one having metachronous (case #3) and the other synchronous tumors (case #38). This is in agreement with earlier reports of Lynch syndrome associated with constitutional methylation 8-10, 13, 19, 25. Regarding family history, three of four patients with constitutional MLH1 methylation presented relatives with Lynch syndrome-associated cancers, but their ages at diagnosis are compatible with sporadic origins. The fourth patient with constitutional MLH1 methylation did not present family history of cancer, something that is quite common when this pathogenic mechanism is operative 8-10, 13, 19, 25.
Methylation of the MLH1 promoter was studied in five regions, including the C- and D-Deng regions. Methylation of the C-Deng region has been directly correlated with transcriptional silencing and loss of MLH1 protein expression 18. The methylation level detected in PBL at the C-Deng region by MS-MLPA and qMSP were below 50% (Table 3), indicating that this alteration might be present in mosaic (the affected allele is not methylated in all cells), as described in other studies 10, 25, 28, 29. In these patients, methylation of the MLH1 gene was also detected in other tissues, like normal colon mucosa (endoderm), oral mucosa (ectoderm), and muscle (mesoderm), representing all three germ layers. Thus, we can infer that in these cases the methylation of MLH1 gene occurred early during embryogenesis 13, 19. Interestingly, we were able to study both the parents and sister of patient #27. None of them presented constitutional MLH1 methylation, suggesting that in this patient MLH1 methylation arose de novo, similar to most cases reported to date 9, 13.
The pattern of transmission of the different forms of epimutations in the MLH1 gene depends on its origin and may be divided into primary and secondary. A dominantly transmitted constitutional MLH1 methylation has been linked to a MLH1 haplotype bearing two single-nucleotide variants: c.-27C>A and c.85G>T (p.Ala29Ser) 25, 30, 31. Studies of the c.−27C>A variant offer the most compelling evidence that MLH1 promoter variants can directly affect the regulation of MLH1 9, 14, 18, 31. This variant has been associated with reduced transcriptional activity and the dominant inheritance of a mosaic constitutional MLH1 methylation 11, 14. In our series this variant linked to secondary MLH1 epimutation was not found. Sanger sequencing of the whole MLH1 promoter (from c.−1469 to intron 1) was performed in PBL from all probands (n = 38) and in the available relatives (n = 3). In one case with MLH1 promoter methylation (#38) the c.−269C>G variant was found in heterozygosity, but the association between these events is not known. No variants (besides common polymorphisms) in the MLH1 promoter were found in the remaining three cases with MLH1 promoter methylation, making it more likely that these patients have primary constitutional methylation, although we cannot exclude the remote possibility that there is a mutation in cis outside the regions studied causing secondary methylation.
When we compared the MLH1 RNA expression in PBL samples (Table 3), we observed that there was significant loss of MLH1 expression in the four cases with constitutional methylation of the MLH1 gene when compared to controls. Our results are in agreement with other studies showing that constitutional methylation causes transcriptional downregulation of the MLH1 gene 19, 25. On the other hand, we took advantage of an heterozygous polymorphism within MLH1 exon 8 (rs1799977) in two patients to determine the effect of the methylation on MLH1 transcriptional activity. In both cases, both alleles were present, but one of them was less expressed when compared with controls. The correlation between MLH1 methylation and expression levels may not be directly proportional, and difficult to measure, given that different approaches, with different sensitivities, were used. However, our RNA studies have demonstrated a decrease in MLH1 gene expression in cases with constitutional methylation, indicating that these events are associated. Recent studies have also shown that MLH1 methylation can present itself as a constitutional alteration that results in the silencing of the affected allele 7, 25, 28, 31-35. Moreover, regarding the information about this polymorphism in patient #27 (heterozygous) and in the parents (father heterozygous and mother homozygous to the A allele), and considering the partial transcriptional silencing of the A allele observed in the cDNA of the proband, we can infer that the methylated allele is inherited from the mother (Fig. 2C). Interestingly, in the majority of sporadic cases reported in the literature, in whom the epimutations has arisen de novo, MLH1 epimutation tended to occur on the maternal allele 7, 8, 28, 29, 34.
In patients with Lynch syndrome, the somatic inactivation, or “second hit”, of the wild-type allele of the affected MMR gene leads to abnormal mismatch repair. This results in the accumulation of errors during DNA replication, especially in repetitive sequences known as microsatellites 34, 36. Consequently, tumors from patients with Lynch syndrome characteristically demonstrate MMR deficiency, defined as the presence of high microsatellite instability (MSI-H) and/or the loss of MMR protein expression, which are the hallmarks of this disorder 37, 38. This feature is present in more than 90% of Lynch syndrome-associated colorectal tumors in general and also in those associated with somatic or constitutional epigenetic silencing of the MLH1 gene 19, 39-41. In fact, we found MSI-H and loss of MLH1 expression, both at the mRNA and protein level, in all four tumors analyzed from the patients with MLH1 constitutional methylation.
The p.Val600Glu BRAF mutation was absent in the tumors of the all four carriers of constitutional MLH1 methylation reported here, as is the case for the majority of the cases in the literature 25, 28, 34, 40, 42. However, occasional reports 16, 28, 43 of BRAF mutation in CRC from patients with MLH1 constitutional methylation exist. Until this issue is clarified in larger series, one should not exclude a patient with an early-onset CRC from MLH1 constitutional methylation testing based on the detection of a BRAF mutation in the tumor, as is currently common practice when selecting patients for MLH1 germline mutation analysis.
We conclude that a significant proportion of patients with Bethesda criteria who have loss of MLH1 protein expression in their tumors and do not have a MLH1 pathogenic germline mutation, display constitutional MLH1 methylation as the mechanism of Lynch syndrome, especially in patients with CRC diagnosed before the age of 50 years, with multiple Lynch syndrome-associated tumors, and no significant family history of early-onset disease. Although the absolute risk of developing Lynch syndrome cancers in patients with MLH1 constitutional methylation and the screening recommendations for relatives are not yet well defined, the inclusion of this analysis in the diagnostic strategy increases the diagnostic yield and allows screening and/or prophylactic measures in more patients with this syndrome. We therefore recommend constitutional MLH1 promoter methylation analysis in all patients diagnosed <50 years of age with tumors presenting MLH1 loss of expression 44.
Acknowledgments
This work was partially supported by IPO Porto Research Center (CI-IPOP) and was awarded the 2015 prize by Grupo de Investigação de Cancro Digestivo (GICD)/Bayer Portugal. JG, RS, and PP are recipients of research scholarships of Liga Portuguesa Contra o Cancro-Núcleo Regional do Norte. MP is a recipient of a postdoc scholarship from Fundação para a Ciência e a Tecnologia (SFRH/BPD/113014/2015).
Conflict of Interest
The authors declare that they have no competing interests.




