Standard pancreatoduodenectomy versus extended pancreatoduodenectomy with modified retroperitoneal nerve resection in patients with pancreatic head cancer: a multicenter randomized controlled trial
Registration number: ChiCTR-TRC-12002548 (ClinicalTrials.gov, http://www.chictr.org.cn).
Abstract
Background
The extent of pancreatoduodenectomy for pancreatic head cancer remains controversial, and more high-level clinical evidence is needed. This study aimed to evaluate the outcome of extended pancreatoduodenectomy (EPD) with retroperitoneal nerve resection in pancreatic head cancer.
Methods
This multicenter randomized trial was performed at 6 Chinese high-volume hospitals that enrolled patients between October 3, 2012, and September 21, 2017. Four hundred patients with stage I or II pancreatic head cancer and without specific pancreatic cancer treatments (preoperative chemotherapy or chemoradiation) within three months were randomly assigned to undergo standard pancreatoduodenectomy (SPD) or EPD, with the latter followed by dissection of additional lymph nodes (LNs), nerves and soft tissues 270° on the right side surrounding the superior mesenteric artery and celiac axis. The primary endpoint was overall survival (OS) by intention-to-treat (ITT). The secondary endpoints were disease-free survival (DFS), mortality, morbidity, and postoperative pain intensity.
Results
The R1 rate was slightly lower with EPD (8.46%) than with SPD (12.56%). The morbidity and mortality rates were similar between the two groups. The median OS was similar in the EPD and SPD groups by ITT in the whole study cohort (23.0 vs. 20.2 months, P = 0.100), while the median DFS was superior in the EPD group (16.1 vs. 13.2 months, P = 0.031). Patients with preoperative CA19–9 < 200.0 U/mL had significantly improved OS and DFS with EPD (EPD vs. SPD, 30.8 vs. 20.9 months, P = 0.009; 23.4 vs. 13.5 months, P < 0.001). The EPD group exhibited significantly lower locoregional (16.48% vs. 35.20%, P < 0.001) and mesenteric LN recurrence rates (3.98% vs. 10.06%, P = 0.022). The EPD group exhibited less back pain 6 months postoperation than the SPD group.
Conclusions
EPD for pancreatic head cancer did not significantly improve OS, but patients with EPD treatment had significantly improved DFS. In the subgroup analysis, improvements in both OS and DFS in the EPD arm were observed in patients with preoperative CA19–9 < 200.0 U/mL. EPD could be used as an effective surgical procedure for patients with pancreatic head cancer, especially those with preoperative CA19–9 < 200.0 U/mL.
Abbreviations
-
- AG
-
- albumin bound paclitaxel plus gemcitabine
-
- AJCC
-
- American Joint Committee on Cancer
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- CA19–9
-
- carbohydrate antigen 19–9
-
- CEA
-
- carcinoembryonic antigen
-
- CSPAC
-
- Chinese Study Group for Pancreatic Cancer
-
- CT
-
- computed tomography
-
- CI
-
- confidence interval
-
- Cr
-
- creatinine
-
- CTA
-
- computed tomography angiography
-
- DFS
-
- disease-free survival
-
- ECOG
-
- Eastern Cooperative Oncology Group
-
- EPD
-
- extended pancreatoduodenectomy
-
- FOLFIRINOX
-
- fluorouracil, leucovorin, irinotecan, and oxaliplatin
-
- HR
-
- hazard ratio
-
- ITT
-
- intention-to-treat
-
- LNs
-
- lymph nodes
-
- MIPD
-
- minimally invasive pancreatoduodenectomy
-
- MD
-
- moderate differentiation
-
- NRS
-
- numerical rating scale
-
- NSAIDs
-
- non-steroidal anti-inflammatory drugs
-
- OPD
-
- open pancreatoduodenectomy
-
- PSM
-
- propensity score matching
-
- PNI
-
- Perineural invasion
-
- PD
-
- poor differentiation
-
- PDAC
-
- pancreatic adenocarcinoma
-
- OS
-
- overall survival
-
- PV
-
- portal vein
-
- RCTs
-
- randomized controlled trials
-
- RBC
-
- red blood cell
-
- RCS
-
- restricted cubic splines
-
- ROC
-
- receiver operating characteristic
-
- SF-36
-
- short form 36
-
- SD
-
- standard deviation
-
- SPD
-
- standard pancreatoduodenectomy
-
- SPSS
-
- Statistical Package For The Social Sciences
-
- SMA
-
- superior mesenteric artery
-
- SMV
-
- superior mesenteric vein
-
- TNM
-
- tumor-node-metastasis
-
- WD
-
- well differentiation
1 INTRODUCTION
Pancreatic cancer is a lethal disease, with a 5-year overall survival (OS) rate of approximately 10% [1]. Concerning the localized disease status, radical resection remains a potentially curable treatment option for patients with clinical stage I to II pancreatic cancer. However, the high risk of local and/or distant recurrence for most resectable diseases makes surgeons dismayed by the value of surgery. In recent decades, several randomized controlled trials (RCTs) on the surgical management of pancreatic cancer have been performed by comparing the outcomes of standard pancreaticoduodenectomy (SPD) to those of extended pancreaticoduodenectomy (EPD). The results showed that, despite its theoretical advantages, EPD does not have survival advantages over standard Whipple surgery [2-6]. Nevertheless, some limitations have made these RCTs unconvincing, such as the small number of enrolled patients, lack of intention-to-treat (ITT) analyses, differential definitions of standard and extended lymphadenectomy, different surgical procedures and techniques among surgeons and institutions, discrepancies in adjuvant treatment, differential definitions of outcome parameters and complications, and inclusion of patients with nonpancreatic ductal adenocarcinoma.
Perineural invasion (PNI) is a pathohistological hallmark of pancreatic cancer and serves as an alternative route for dissemination in addition to the lymphatic and vascular systems [7]. However, a consensus on the extent of nerve plexus dissection in pancreatoduodenectomy has not been reached.
Due to the ominous features of PNI in pancreatic ductal adenocarcinoma (PDAC), the lack of a surgical consensus and limited results from previous RCTs, we performed this randomized controlled study to compare the outcomes between SPD and EPD with retroperitoneal nerve resection in patients with pancreatic head cancer.
2 METHODS
2.1 Participating hospitals
Initially, 3 hospitals agreed to participate in this study. Then, 3 other centers showed interest in participating and joined the study one year later. Ultimately, patients were enrolled from 6 tertiary hospitals (all are high-volume centers in pancreatic cancer diagnosis and treatment in China, Supplementary Table S1).
2.2 Patients and study design
This multicenter, randomized, controlled, nonblinded, parallel-group trial compared SPD versus EPD with retroperitoneal nerve resection for pancreatic head cancer. The protocol complied with the Declaration of Helsinki and was approved and overseen by the clinical ethics committee of each participating hospital. This study was registered at the Chinese Clinical Trial Registry (http://www.chictr.org.cn, no: ChiCTR-TRC-12002548). Patients with resectable pancreatic head tumors eligible to undergo pancreatoduodenectomy were enrolled between October 3, 2012, and September 21, 2017. All listed authors had access to the study data and approved the final manuscript. The study sponsors had no role in the design and conduct of the study.
2.3 Inclusion and exclusion criteria
Patients were included if they (1) were 18 to 80 years of age (the upper limit age was changed from 70 to 80 years due to an increase in the number of adults 70 years and older, and pancreatic cancer tends to occur at an older age), regardless of gender; (2) had potentially curable cancer of the pancreatic head (stage I or II according to the American Joint Committee on Cancer [AJCC] 7th edition), as shown on preoperative imaging examinations (enhanced computed tomography, magnetic resonance imaging/magnetic resonance cholangiopancreatography, endoscopic ultrasound, endoscopic retrograde cholangiopancreatography, positron emission tomography-computed tomography or fine-needle aspiration biopsy); (3) had a Karnofsky performance status score > 70; (4) had Loyer grade A to D type; (5) had no obvious surgical contraindications; and (6) provided written informed consent. Patients were excluded if they (1) had an unresectable condition or metastasis (stage III or IV) found during surgery; (2) had a pathologic diagnosis of a benign tumor of the head of the pancreas or a tumor in the tail of the pancreas; (3) had surgical contraindications; (4) had a history of other malignancies; (5) were pregnant (urine human chorionic gonadotropin (HCG) > 2500 IU/L, diagnosed as early pregnancy), planned to become pregnant or were lactating; (6) had received other specific pancreatic cancer treatments (preoperative chemotherapy or chemoradiation) within three months; (7) had mental disease; (8) participated in other clinical trials 3 months before; or (9) had impaired visceral function (cardiac function 3-4, alanine aminotransferase[ALT] and/or aspartate aminotransferase [AST] exceeding 3 times the upper limit, creatinine [Cr] beyond the upper limit).
2.4 Study treatment
In the SPD group, lymph nodes (LNs) around the gastric pylorus (stations 5, 6), LNs around the pancreatic head (stations 13a, 13b, 17a, and 17b), LNs anterior to the common hepatic artery (station 8a), LNs on the right side of the hepatoduodenal ligament (stations 12b, 12c), and LNs on the right side of the root of the superior mesenteric artery (SMA) (stations 14a, 14b) were dissected without retroperitoneal nerve resection. During EPD, the following nerve tissues at the retroperitoneum and LNs around the pancreatic head were dissected: (I) nerves and soft tissues between the inferior vena cava (including the aortic plexus) and abdominal aorta (including LN stations 16a2 and 16b1); (II) all the nerves and soft tissues around the hepatoduodenal ligament, which were completely dissected and skeletonized (including whole LN station 12); (III) the common hepatic artery, which was isolated, and its surrounding nerves and soft tissues (including LN stations 8a and 8p); (IV) the celiac trunk, which was isolated, and its nerves and soft tissues 270° around the right longitudinal axis (including LN station 9); (V) the root of the SMA, which was dissected to open the vascular sheath, and the proximal uncinate process mesentery, which was removed along its right side, the nerves and soft tissues 270° around the right longitudinal axis (including LN stations 14a and 14b); and (VI) the nerves and soft tissues in the dense postpancreatic connective tissues that fixed the pancreas at the celiac trunk-aorta-SMA artery axis. Both the portal vein (PV) and the superior mesenteric vein (SMV) were resected and reconstructed in patients with PV/SMV involvement to achieve radical resection. Differences in the extent of resection are summarized in Supplementary Table S2 and Figure 1.
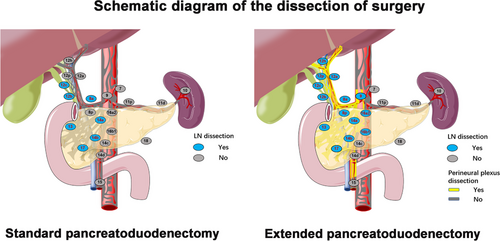
First, all participating hospitals were high-volume pancreatic surgery centers (completing more than 100 pancreatoduodenectomy operations each year), and all the surgeons participating in this trial had rich surgical experience (the cumulative number of pancreatoduodenectomies exceeded 300 surgeries). Second, for the homogenization of the surgical technique, the Pancreatic Cancer Committee of the Chinese Anti-Cancer Association conducted multiple unified training sessions and assessments on the operators before the start of the study [8]. Specifically, (1) unified surgical specifications and procedures were formulated; (2) strict assessment of EPD and SPD surgical proficiency from each operator by independent expert evaluation was performed; (3) 6 meetings (April 15, May 20, June 24, July 15, August 12 and September 15, 2012) were held to discuss the surgical videos and unify the surgical procedures before patient enrollment; and (4) all randomized cases were evaluated for heterogeneity through objective data, such as surgical images (Supplementary Figure S1) and videos, by third-party experts to ensure homogeneity of patient enrollment.
2.5 Randomization
Patients were randomly assigned at a 1:1 ratio to undergo SPD or EPD with retroperitoneal nerve resection using a stratified permuted block method. Patients were stratified according to the coordinating hospitals and predefined preoperative cancer antigen 19-9 (CA19-9) (< 200.0 U/mL or ≥ 200.0 U/mL). Computer-generated random assignment lists were created at the School of Public Health, Sun Yat-sen University (Guangzhou, Guangdong, China). The assignments were placed in sealed envelopes, labeled by stratum, and unsealed only after exploratory laparotomy. Investigators at each center enrolled participants and assigned them to their corresponding interventions. Each patient received a unique study number that remained unchanged throughout the trial.
2.6 Study outcomes
The primary endpoint of this study was OS by ITT, calculated from the date of randomization until the date of death from any cause. The secondary endpoints were disease-free survival (DFS), mortality, morbidity, and postoperative pain intensity. DFS was calculated from the date of randomization until the date of the first event of either recurrence or death from any cause. The censored data were defined as patients without events at study termination or those lost to follow-up at any time during the study. Prespecified subgroups were defined for preoperative CA19–9 (< 200.0 U/mL or ≥ 200.0 U/mL based on the predictive value of CA19–9 from previous reports [9-11] and discussions of the researchers), and we performed subgroup analyses with this cutoff for OS and DFS. Moreover, we performed post hoc subgroup analyses of other factors, including age (< 60/≥ 60), gender (male/female), preoperative carcinoembryonic antigen (CEA) (< 5.00/≥ 5.00 ng/mL), PV/SMV resection (yes/no), type of resection (R0/R1), N stage (N0/N1), tumor-node-metastasis (TNM) stage (I/IIA/IIB), histology (well differentiation [WD] and moderate differentiation [MD]/poor differentiation [PD]), perineural invasion (+/-) and adjuvant treatment (yes/no), for OS and DFS in the ITT population. Pain intensity was assessed with a numerical rating scale (NRS) [12]. An 11-point scale from 0 to 10 was used to describe pain (minimum to maximum). The NRS was explained to the patients, and they were asked to circle the number best representing the degree of their pain on the last day of preoperation (baseline, day 0), 1 month postoperation, and 6 months postoperation. The patients were followed up once a month from 1 to 6 months after surgery, every 3 months from 6 months to 3 years after surgery, and every 6 months for 3 years after surgery. The follow-up examinations included routine blood tests, biochemical tests, digestive tract tumor index detection, B-ultrasound, and enhanced computed tomography (CT) scan examinations. The short form 36 (SF-36) was used to assess the patients’ general health status. The SF-36 is composed of eight multi-item scales (physical functioning, physical role, pain, general health, vitality, social function, emotional role, and mental health), with scores for each of these scales (or dimensions) ranging from 0 to 100, whereby a higher score indicates a higher health-related quality of life [13]. The patients completed the SF-36 questionnaire with the assistance of the follower. The SF-36 score and pain score of the patients were assessed preoperatively and every 3 months after surgery. Each follow-up included gathering information on the chemotherapy regimen, adjuvant therapy regimen and adverse reactions. The last date of follow-up was defined as three calendar years since the last enrolled patient underwent surgery.
An interim analysis was conducted on the primary endpoint when 50% of patients were randomized and completed the 6-month follow-up. The interim analysis was performed by an independent statistician blinded to the treatment allocation. The trial was calculated to end using symmetric stopping boundaries at P < 0.001.
2.7 Pathological analysis
The LNs and nerve tissues removed during dissection were marked with the exact location and sent for a pathological examination after resection. Pathology reports contained the primary pathologic diagnosis, the extent of disease, margin status, LN status and overall pathological stage as previously described. The stages of the resected specimens were classified according to the AJCC TNM classification (7th edition). All participating surgeons observed intraoperative photographs or videos of both groups to ensure consistency of the extent of nerve, tissue and LN dissection.
2.8 Postoperative chemotherapy
Chemotherapy was recommended for all patients except for those with a poor performance status (grade 3-5 by ECOG [Eastern Cooperative Oncology Group] performance status scale) [14] or organ dysfunction and those who refused adjuvant treatment. The first postoperative chemotherapy cycle started within 2 months of surgery. The chemotherapy regimen was as follows: gemcitabine 1000 mg/m2, dissolved in 100 mL normal saline, intravenous infusion, completed within 30 minutes; once weekly, after 2 consecutive times, rest for 1 week, every 3 weeks for a course of treatment, for 8 consecutive courses.
2.9 Postoperative pain control strategy
In this study, for postoperative pain control, all our patients followed the principle of pain ladder treatment. For mild pain, we recommended using nonopioid adjuvant analgesics (ibuprofen, aspirin). For moderate pain, we recommended using weak opioids plus or minus nonsteroidal anti-inflammatory drugs (NSAIDs) plus or minus adjuvant pain relievers (tramadol, celebrex, codeine, etc.). For severe pain, we recommended using opioids plus or minus nonsteroidal adjuvant painkillers (morphine, duloxetine, etc.)
2.10 Sample size and statistical analysis
Based on previous RCTs [3, 6, 15] and our pre-experiment analysis, our trial was powered for the superiority of OS data at 3 years according to the operation, assuming that the 3-year OS rate (35%) of patients from the EPD group was 15% higher than that of patients from the SPD group (20%). Our estimation showed that an enrollment of 180 patients would provide 80% power to detect the superiority of a procedure with a 1-sided α = 0.05 and β = 0.2. Taking into account an estimated 10% dropout rate, the sample size was increased to 200 participants per group (n = 400).
The ITT population was defined as all randomly assigned patients. The qualified population was defined as patients enrolled in randomization who underwent appropriate surgery without rule violation for the extent of surgical dissection, with ductal adenocarcinoma as the final pathology, with a complete case report form and without loss to follow-up. Analyses were conducted using the ITT principle, irrespective of any protocol deviations or violations.
Patients who underwent the operation to which they were originally allocated and satisfied the criteria for optimal surgery based on photographs uploaded to our data center were evaluated. Categorical variables are expressed as proportions, whereas continuous variables are expressed as the medians (range, minimum to maximum) or means (standard deviation, SD) where appropriate. Missing data were handled using a Markov chain Monte Carlo multiple imputation approach with the assumption of missing data at random [16]. Continuous variables were reported as the median and range, where appropriate, and compared using Student's t-test (when the data conformed to a normal distribution) or Mann‒Whitney U test (when the data did not conform to a normal distribution). Nominal data were compared using χ2 tests or Fisher's exact test. Survival outcomes were calculated using the Kaplan‒Meier method and compared using the log-rank test (stratified for predefined preoperative CA19–9 [< 200.0 U/mL or ≥ 200.0 U/mL]). Variables revealed as statistically significant by the univariate analysis were included in the multivariate analysis, which was performed using a Cox proportional hazards regression model. Both univariate and multivariate analyses were performed for the treatment method. To evaluate the association between levels of CA19–9 and disease-progression survival, a multivariable Cox model with restricted cubic splines (RCS) was built. RCS has been widely described as a valid strategy for analyzing the relationship between survival and independent variables [17, 18]. The analysis was performed with R software version 4.2.1. To minimize the effect of confounding factors and potential bias between the SPD and EPD groups, the propensity score was calculated using logistic regression, and we performed 1:1 patient matching by the nearest-neighbor matching method without replacement. We used a caliper radius equal to 0.2 of the standard deviation to prevent poor matching. The variables included in the matching model were age, gender, preoperative CA19–9 value, PV/SMV resection, N stage and T stage. All statistical analyses were performed using Statistical Package For The Social Sciences (SPSS) version 23.0 (SPSS Inc., Chicago, IL, USA), and a 2-sided P value less than 0.05 was considered statistically significant.
2.11 Data management
After random assignment, the following data were collected from all patients: clinical and pathological information, details of the operative procedure (including photographs of the operation field and a surgeon questionnaire detailing the operative findings), and other relevant information. Adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE, version 4.0). Follow-up was obtained from hospitalization or outpatient records, telephone calls, and assistance from the Chinese public security administration. Assessors were blinded to the treatment groups (i.e., the SPD group vs. the EPD group).
3 RESULTS
3.1 Patients
Between October 3, 2012, and September 21, 2017, 468 patients from six Chinese centers were screened (Figure 2). After enrollment, 68 patients were excluded for the following reasons: 28 patients refused to participate despite initially agreeing, 32 patients had unresectable or metastatic tumors during the intraoperative exploration, and 8 patients actually received distal pancreatectomy. Thus, 400 eligible patients were enrolled and randomly allocated into two groups (Figure 2). The last follow-up date was expected to be on September 21, 2020, but to further improve the integrity of the data, we extended the follow-up time to November 30, 2020.
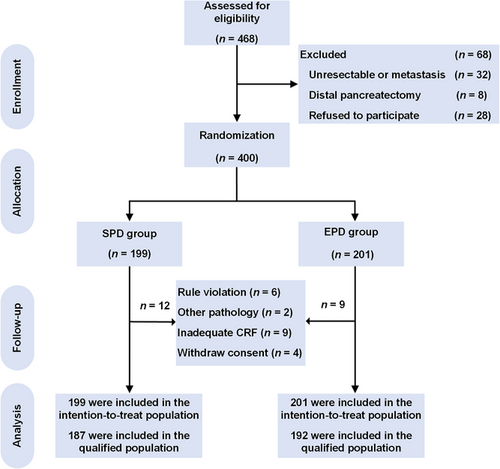
Study flowchart illustrating patients’ randomization and group allocation for treatments with SPD and EPD.
Abbreviations: SPD, standard pancreatoduodenectomy; EPD, extended pancreatoduodenectomy; CRF, case report form.
Of the remaining 400 patients, 199 were randomized to receive SPD, 201 were randomized to receive EPD with retroperitoneal nerve resection and constituted the ITT population. Of them, 21 were excluded for the following reasons: (1) having undergone inadequate or inappropriate surgery, as revealed by reviewing the images of the surgical field (n = 6); (2) having a nonductal adenocarcinoma (n = 2), such as ampullary adenocarcinoma and duodenal adenocarcinoma; or (3) having inadequate case report forms (n = 9) or withdrawing consent (n = 4, two by the patients and two by the surgeons). Consequently, 379 qualified participants were included, with 187 in the SPD group and 192 in the EPD group. The two groups were well balanced in the following characteristics at baseline: median age, gender, preoperative CEA and CA19–9 levels, preoperative total bilirubin, and resection rate of the PV/SMV (Table 1). The operation time was 45 minutes longer in the EPD group than in the SPD group (P < 0.001), with no difference in either estimated blood loss (EPD vs. SPD, median [range, mL], 300 [20–2,500] vs. 300 [20–6,000], P = 0.113; Table 1) or red blood cell (RBC) transfusion (P = 0.870; Table 1). Overall, 343 patients underwent adjuvant treatment after surgical resection (Table 1).
| Clinical variables | SPD group | EPD group | P value |
|---|---|---|---|
| Age, median (range), year | 60 (28-79) | 59 (19-79) | 0.916 |
| Gender, male/female, male% | 111/88, 55.78% | 117/84, 58.21% | 0.686 |
| Preoperative CEA, median (range), ng/mL | 3.37 (0.33-1256.00) | 3.30 (0.20-422.40) | 0.659 |
| Preoperative CA19–9, median (range), U/mL | 187.2 (0.6-12000.0) | 180.5 (0.6-15968.0) | 0.769 |
| Preoperative total bilirubin, median (range), μmol/L | 91.8 (4.4-599.2) | 94.7 (0.9-637.4) | 0.515 |
| OP time, median (range), min | 330 (180-1031) | 375 (240-625) | < 0.001 |
| EBL, median (range), mL | 300 (20-6000) | 300 (20-2500) | 0.113 |
| Transfusion (RBC pack), median (range), unit | 0 (0-25.5) | 0 (0-8.0) | 0.870 |
| PV/SMV resection, cases (%) | 44 (22.11) | 41 (20.40) | 0.715 |
| Pathologic variables | |||
|---|---|---|---|
| R1 resection, cases (%) | 25 (12.56) | 17 (8.46) | 0.195 |
| Histology differentiation, cases (%) | 0.109 | ||
| Well | 8 (4.02) | 14 (6.97) | |
| Moderate | 94 (47.24) | 108 (53.73) | |
| Poor | 97 (48.74) | 79 (39.30) | |
| Tumor size, median (range), cm | 3.2 (0.8-10.0) | 3.0 (0.3-8.0) | 0.400 |
| T stage, cases (%) | 0.762 | ||
| T1 | 7 (3.52) | 10 (4.98) | |
| T2 | 21 (10.55) | 20 (9.95) | |
| T3 | 171 (85.93) | 171 (85.07) | |
| LN (+), cases (%) | 105 (52.76) | 123 (61.19) | 0.106 |
| Total retrieved LNs, median (range) | 15 (7-46) | 20 (7-63) | < 0.001 |
| No. positive LNs, median (range) | 1 (0-11) | 1 (0-29) | 0.005 |
| LN (+) ratio, median (range) | 0.06 (0-0.75) | 0.07 (0-0.91) | 0.101 |
| TNM stage*, cases (%) | 0.106 | ||
| IA | 4 (2.01) | 7 (3.48) | |
| IB | 9 (4.52) | 12 (5.97) | |
| IIA | 81 (40.70) | 59 (29.35) | |
| IIB | 105 (52.76) | 123 (61.19) | |
| Adjuvant treatment, cases (%) | 173 (86.93) | 170 (84.58) | 0.568 |
| Complete adjuvant treatment, cases (%) | 130 (65.33) | 125 (62.19) | 0.534 |
- * The stages of the resected specimens were classified according to the AJCC TNM classification (7th edition), I/IIB and IIA/IIB in the TNM stage are equivalent to N0/N1, so multivariate analysis is not included.
- Abbreviations: ITT, intention-to-treat; SPD, standard pancreatoduodenectomy; EPD, extended pancreatoduodenectomy; CEA, carcinoembryonic antigen; CA19–9, carbohydrate antigen 19–9; OP, operation; EBL, estimated blood loss; RBC indicates red blood cells; PV, portal vein; SMV, superior mesenteric vein; LN, lymph node; pancre1atoduodenectomy; AJCC, American Joint Committee on Cancer.
3.2 Postoperative pathological differences between groups
Table 1 also shows the pathological characteristics of the two groups. The percentage of patients who underwent R1 resection was similar in the SPD group (12.56%) and the EPD group (8.46%; P = 0.195). In addition, there were no differences between the two groups in tumor size, T stage or AJCC TNM stage. As expected, the total number of retrieved LNs was significantly higher in the EPD group (median [range], 20 [7–63]) than in the SPD group (median [range], 15 [7–46]; P < 0.001). Moreover, a significantly higher number of positive LNs was observed in the EPD group than in the SPD group (P = 0.005), but the LN (+) ratio was not significantly different between the two groups (P = 0.101).
3.3 Morbidity and mortality
Although there was a trend for higher morbidity in the EPD group, there was no significant difference between the two groups (EPD vs. SPD, 37.81% vs. 34.17%; P = 0.467; Table 2). Moreover, no significant difference was found between the two groups in postoperative hospital stay (EPD vs. SPD, median [range], 18 [6-105] vs. 18 [9-49], P = 0.232; Supplementary Table S3). The incidence of diarrhea in the EPD group within 3 months of surgery was 7.46% compared with 5.03% in the SPD group; no significant difference was found (P = 0.409), suggesting that dissection of the retroperitoneal plexus 270° on the right side surrounding the celiac trunk and SMA might not significantly affect bowel movement. The inpatient mortality was similar between the two groups (EPD vs. SPD, 0.50% vs. 0.50%, Table 2). Regarding the specific cause of death, 1 patient in the EPD group died of severe sepsis with gastroduodenal artery rupture, and 1 patient in the SPD group died of cirrhotic liver failure.
| Outcome | SPD(n = 199) | EPD(n = 201) | HR (95% CI) | OR (95% CI) | P value |
|---|---|---|---|---|---|
| Primary | |||||
| Median OS, months | 20.2 | 23.0 | 0.84 (0.68 to 1.04) | N/A | 0.100 |
| Secondary | |||||
| Median DFS, months | 13.2 | 16.1 | 0.80 (0.66 to 0.98) | N/A | 0.031 |
| OS rate, % | |||||
| At 1 year | 70.35 | 76.62 | N/A | 0.72 (0.46 to 1.12) | 0.174 |
| At 2 year | 40.20 | 45.77 | N/A | 0.80 (0.54 to 1.17) | 0.268 |
| At 3 year | 22.61 | 29.35 | N/A | 0.70 (0.44 to 1.10) | 0.139 |
| At 5 year | 9.05 | 9.95 | N/A | 0.90 (0.46 to 1.73) | 0.865 |
| Safety, n (%) | |||||
| Morbidity | 68 (34.17) | 76 (37.81) | N/A | 1.17 (0.78 to 1.78) | 0.467 |
| Mortality | 1 (0.50)* | 1 (0.50)** | N/A | 0.99 (0.05 to 18.90) | NS |
| Mean (SD)*** | |||||
| Abdominal pain upper changing in 1 month from baseline**** | -2.05 (1.90) | -1.77 (2.09) | N/A | 0.28 (-0.11 to 0.67) | 0.163 |
| Back pain changing in 1 month from baseline | -0.77 (1.71) | -0.77 (1.72) | N/A | 0.02 (-0.33 to 0.37) | 0.987 |
| Abdominal pain upper changing in 6 months from baseline***** | -0.90 (3.12) | -0.70 (2.97) | N/A | 0.20 (-0.43 to 0.82) | 0.533 |
| Back pain changing in 6 months from baseline | 0.48 (2.83) | -0.35 (2.43) | N/A | -0.82 (-1.37 to -0.29) | 0.003 |
- * One died of cirrhotic liver failure.
- ** One died of severe sepsis with gastroduodenal artery rupture.
- *** Data of pain intensity were shown as mean (standard deviation).
- **** The data was calculated as pain intensity in 1 month postoperation minus pain intensity baseline. The baseline representsthe last day of preoperation.
- ***** The data was calculated as pain intensity in 6 months postoperation minus pain intensity baseline.
- Abbreviations: ITT, intention-to-treat; SPD, standard pancreatoduodenectomy; EPD, extended pancreatoduodenectomy; HR, hazard ratio; CI, confidence interval; OS, overall survival; DFS, disease-free survival; OR, odds ratio; NS, not significant; NRS, numerical rating scale; SD, standard deviation.
3.4 Survival and recurrence data
For the ITT analysis, all randomly enrolled patients were included. Kaplan‒Meier analysis of OS showed a better prognostic trend for the EPD group than for the SPD group, but the difference was not significant (Figure 3A). The median OS (ITT population) was 23.0 months in the EPD group versus 20.2 months in the SPD group (hazard ratio (HR), 0.84; 95% CI, 0.68 to 1.04; P = 0.100; Table 2). The OS rates in the EPD and SPD groups were 76.62% and 70.35% at 1 year, 45.77% and 40.20% at 2 years, 29.35% and 22.61% at 3 years, and 9.95% and 9.05% at 5 years, respectively (Table 2). DFS was significantly better in the EPD group by ITT (EPD vs. SPD, 16.1 months vs. 13.2 months; HR, 0.80; 95% CI, 0.66 to 0.98; P = 0.031; Figure 3B and Table 2).
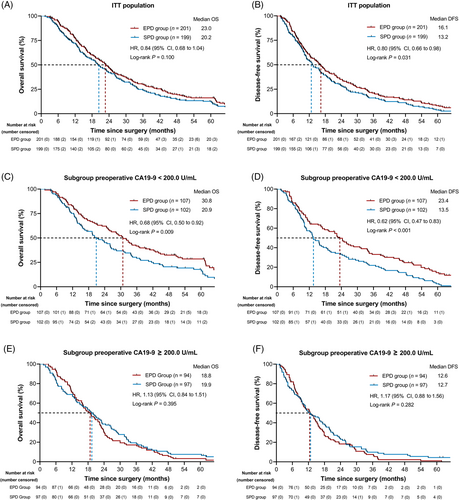
The outcome of the SPD and EPD groups in the ITT population. (A) OS and (B) DFS in the ITT population. (C) OS and (D) DFS in the ITT population for the prespecified subgroup of preoperative CA19–9 < 200.0 U/mL. (E) OS and (F) DFS in the ITT population for the prespecified subgroup of preoperative CA19–9 ≥ 200.0 U/mL.
Abbreviations: SPD, standard pancreatoduodenectomy; EPD, extended pancreatoduodenectomy; OS, overall survival; DFS, disease-free survival; ITT, intention-to-treat; CA19–9, carbohydrate antigen 19–9; HR, hazard ratio; CI, confidence interval.
3.5 Subgroup analysis
The predefined subgroup with a preoperative CA19–9 < 200.0 U/mL showed a significantly improved median OS and DFS for EPD (EPD vs. SPD; OS: 30.8 months vs. 20.9 months; HR, 0.68; 95% CI, 0.50 to 0.92; P = 0.009; DFS: 23.4 months vs. 13.5 months; HR, 0.62; 95% CI, 0.47 to 0.83; P < 0.001, Figure 3C-D and Table 3). In the subgroup with a preoperative CA19–9 < 200.0 U/mL, the respective 1-, 2-, 3-, and 5-year OS rates were 82.24%, 59.81%, 40.19% and 16.82% in the EPD arm and 72.55%, 42.16%, 26.47%, and 10.78% in the SPD arm (Table 3). The predefined subgroup of patients with a preoperative CA19–9 ≥ 200.0 U/mL showed no significant difference in OS or DFS (Figure 3E-F and Table 4). Post hoc analysis of subgroups by age (< 60/≥ 60), gender (male/female), PV/SMV resection (yes/no), preoperative CEA (< 5.00/≥ 5.00 ng/mL), resectability (resectable/borderline resectable), type of resection (R0/R1), N stage (N0/N1), TNM stage (I/IIA/IIB), perineural invasion (positive/negative) and postoperative adjuvant treatment (with/without) showed no differences in OS (Supplementary Table S4). In addition, in the subgroup with well and moderate pathological differentiation, the EPD group showed superior oncologic outcomes in terms of median OS and DFS (OS: HR, 0.72; 95% CI, 0.54 to 0.96, P = 0.020, Supplementary Table S4; DFS: HR, 0.69; 95% CI, 0.52 to 0.91, P = 0.006, Supplementary Table S5). In the subgroup analysis (Supplementary Table S5), with the results of some prognostic factors presented in Supplementary Figure S2 and Supplementary Figure S3, survival benefits for DFS were found from EPD treatment in patients with preoperative CEA < 5.00 ng/mL (HR, 0.75; 95% CI, 0.59 to 0.96, P = 0.018, Supplementary Figure S2B), without PV/SMV resection (HR, 0.79; 95% CI, 0.63 to 0.99, P = 0.038, Supplementary Figure S2F), with a resectable stage (HR, 0.74; 95% CI, 0.58 to 0.94, P = 0.013, Supplementary Figure S3H), with R0 resection (HR, 0.77; 95% CI, 0.62 to 0.95, P = 0.014, Supplementary Figure S2H), and with postoperative adjuvant chemotherapy (HR, 0.77; 95% CI, 0.62 to 0.96, P = 0.019, Supplementary Figure S2J).
| Outcome | SPD(n = 102) | EPD(n = 107) | HR (95% CI) | OR (95% CI) | P value |
|---|---|---|---|---|---|
| Primary | |||||
| Median OS, months | 20.9 | 30.8 | 0.68 (0.50 to 0.92) | N/A | 0.009 |
| Secondary | |||||
| Median DFS, months | 13.5 | 23.4 | 0.62 (0.47 to 0.83) | N/A | <0.001 |
| OS rate, % | |||||
| At 1 year | 72.55 | 82.24 | N/A | 0.57 (0.30 to 1.11) | 0.100 |
| At 2 year | 42.16 | 59.81 | N/A | 0.49 (0.28 to 0.84) | 0.013 |
| At 3 year | 26.47 | 40.19 | N/A | 0.54 (0.29 to 0.94) | 0.041 |
| At 5 year | 10.78 | 16.82 | N/A | 0.60 (0.28 to 1.35) | 0.234 |
| Safety, n (%) | |||||
| Morbidity | 38 (37.25) | 34 (31.78) | N/A | 0.78 (0.45 to 1.36) | 0.467 |
| Mean (SD)* | |||||
| Abdominal pain upper changing in 1 month from baseline** | -2.11(1.80) | -1.78(2.01) | N/A | 0.33 (-0.19 to 0.85) | 0.210 |
| Back pain changing in 1 month from baseline | -0.63(1.56) | -0.71(1.61) | N/A | -0.07 (-0.51 to 0.36) | 0.740 |
| Abdominal pain upper changing in 6 months from baseline*** | -0.89(3.09) | -1.01(2.74) | N/A | -0.11 (-0.93 to 0.71) | 0.782 |
| Back pain changing in 6 months from baseline | 0.59(2.93) | -0.29(2.32) | N/A | -0.89 (-1.63 to -0.14) | 0.020 |
- * Data of pain intensity were shown as mean (standard deviation).
- ** The data was calculated as pain intensity in 1 month postoperation minus pain intensity baseline.
- *** The data was calculated as pain intensity in 6 months postoperation minus pain intensity baseline.
- Abbreviations: ITT, intention-to-treat; SPD, standard pancreatoduodenectomy; EPD, extended pancreatoduodenectomy; CA19–9, carbohydrate antigen 19–9; HR, hazard ratio; CI, confidence interval; OS, overall survival; DFS, disease-free survival; OR, odds ratio; NS, not significant; NRS, numerical rating scale; SD, standard deviation.
| Outcome | SPD(n = 97) | EPD(n = 94) | HR (95% CI) | OR (95% CI) | P value |
|---|---|---|---|---|---|
| Primary | |||||
| Median OS, months | 19.9 | 18.8 | 1.13 (0.84 to 1.51) | N/A | 0.395 |
| Secondary | |||||
| Median DFS, months | 12.7 | 12.6 | 1.17 (0.88 to 1.56) | N/A | 0.282 |
| OS rate, % | |||||
| At 1 year | 68.04 | 70.21 | N/A | 0.90 (0.50 to 1.63) | 0.757 |
| At 2 year | 38.14 | 29.79 | N/A | 1.45 (0.79 to 2.60) | 0.285 |
| At 3 year | 18.56 | 17.02 | N/A | 1.11 (0.54 to 2.35) | 0.851 |
| At 5 year | 7.22 | 2.13 | N/A | 3.58 (0.76 to 17.32) | 0.170 |
| Safety, n (%) | |||||
| Morbidity | 30 (30.93) | 42 (44.68) | N/A | 1.80 (1.01 to 3.32) | 0.054 |
| Mean (SD)* | |||||
| Abdominal pain upper changing in 1 month from baseline** | -1.99(2.01) | -1.77(2.18) | N/A | 0.22 (-0.38 to 0.82) | 0.462 |
| Back pain changing in 1 month from baseline | -0.91(1.85) | -0.84(1.85) | N/A | 0.08 (-0.45 to 0.60) | 0.774 |
| Abdominal pain upper changing in 6 months from baseline*** | -0.91(3.18) | -0.35(3.19) | N/A | 0.56 (-0.41 to 1.53) | 0.256 |
| Back pain changing in 6 months from baseline | 0.35(2.72) | -0.41(2.55) | N/A | -0.76 (-1.56 to 0.04) | 0.064 |
- * Data of pain intensity were shown as mean (standard deviation).
- ** The data was calculated as pain intensity in 1 month postoperation minus pain intensity baseline.
- *** The data was calculated as pain intensity in 6 months postoperation minus pain intensity baseline.
- Abbreviations: ITT, intention-to-treat; SPD, standard pancreatoduodenectomy; EPD, extended pancreatoduodenectomy; CA19–9, carbohydrate antigen 19–9; HR, hazard ratio; CI, confidence interval; OS, overall survival; DFS, disease-free survival; OR, odds ratio; NS, not significant; NRS, numerical rating scale; SD, standard deviation.
As mentioned in the methods section, the prespecified threshold of the subgroup for CA19–9 was 200.0 U/mL. The rationale for this threshold was examined through receiver operating characteristic (ROC) and RCS analyses. The ROC analysis identified that the best cutoff value of preoperative CA19–9 was 198.7 U/mL for predicting tumor DFS, a value very close to 200.0 U/mL (Figure 4A-B); in addition, as shown in the RCS model (Figure 4C), the HR values for DFS in cases with CA19–9 ≥ 200.0 U/mL were consistently greater than those for cases with CA19–9 < 200.0 U/mL, both supporting the prespecified threshold of 200.0 U/mL.
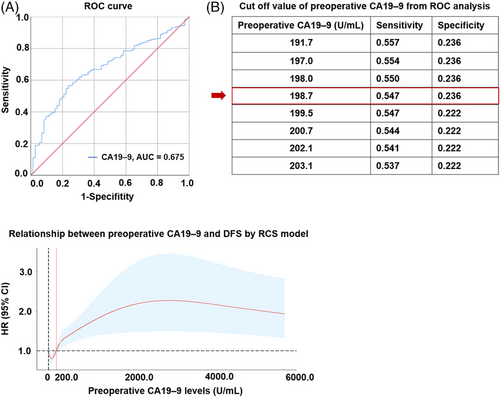
Analysis for the best cutoff value of preoperative CA19–9. (A) ROC curve for preoperative CA19–9. The red diagonal represents sensitivity plus specificity = 1. The blue polyline represents the ROC curve of preoperative CA 19–9 in predicting DFS. (B) The cutoff value of preoperative CA19–9 from ROC analysis. (C) Relationship between preoperative CA19–9 levels and DFS by RCS model. The red curve represents the result of RCS, which indicates the HR value of CA19–9 on DFS. The black vertical dotted line indicates the zero value of CA19–9; the black horizontal dotted line represents the reference HR value of 1.0; the red vertical dotted line indicates the position of CA19–9 of 200.0 U/mL.
Abbreviations: CA19–9, carbohydrate antigen 19–9; ROC, receiver operating characteristic; AUC, area under the curve; DFS, disease-free survival; RCS, restricted cubic splines; HR, hazard ratio; CI, confidence interval.
To evaluate the benefit of adjuvant chemotherapy according to different CA19–9 levels, we compared the prognosis of patients stratified by adjuvant chemotherapy application in the patients with CA19–9 < 200.0 U/mL and those with CA19–9 ≥ 200.0 U/mL. In the subgroup with preoperative CA19–9 < 200.0 U/mL, there was no difference in OS and DFS between those receiving or not receiving adjuvant chemotherapy. In contrast, in the subgroup with preoperative CA19–9 ≥ 200.0 U/mL, adjuvant chemotherapy improved both OS and DFS (Supplementary Table S6). Since DFS benefits were found from EPD treatment for patients who received adjuvant chemotherapy (Supplementary Table S5), subgroup analyses were further performed to evaluate whether different CA19–9 levels could affect the benefits of adjuvant chemotherapy. In the subgroup with preoperative CA19–9 < 200.0 U/mL, a significant improvement in median OS and DFS was found in the EPD group in patients with adjuvant chemotherapy (Supplementary Table S7). However, in the subgroup with preoperative CA19–9 ≥ 200.0 U/mL, EPD showed no advantage regardless of whether patients received adjuvant chemotherapy (Supplementary Table S8). In addition, we conducted a 1:1 propensity matching analysis and further performed survival and subgroup analyses for the treatments after matching. Consistent with our aforementioned findings, no difference was found in the propensity score matching (PSM) population between the treatment groups in both OS and DFS (Supplementary Table S9), while patients with predefined preoperative CA19–9 < 200.0 U/mL showed a better outcome in the EPD group (Supplementary Table S10). In terms of the subgroup analysis in the PSM population, EPD treatment significantly improved the OS in patients with WD & MD (Supplementary Table S11) and DFS in patients with preoperative CEA < 5.00 ng/mL, with resectable stage, with R0 resection or with WD & MD (Supplementary Table S12).
3.6 Prognostic factors
Preoperative CEA ≥ 5.00 ng/mL, preoperative CA19–9 ≥ 200.0 U/mL, PV/SMV resection, borderline resectable, R1 resection, N1 stage, poor differentiation, and absence of adjuvant treatment were identified as adverse prognostic factors for OS in the univariate analysis (ITT population, Supplementary Table S13). When these factors combined with treatment methods were included in the multivariate Cox proportional hazards model, preoperative CA19–9 ≥ 200.0 U/mL, N1 stage and poor differentiation remained indicators for poor OS (Supplementary Table S13).
In the EPD group, the retroperitoneal nerve plexus was removed during the surgery and marked with the exact location for a separate pathological examination. The univariate analysis of OS (qualified population in the EPD group) showed no significant difference in patients with or without nerve invasion in celiac axis plexus, SMA plexus, hepatoduodenal ligament plexus, common hepatic artery plexus or post-pancreatic plexus. In particular, nerve invasion of the aortic plexus suggested poor OS (HR, 0.58; 95% CI, 0.36 to 0.94; P = 0.006; Supplementary Table S14).
3.7 Recurrence pattern and pain intensity
There were no differences in the total recurrence rate between the EPD and SPD groups (Supplementary Table S15). As expected, the locoregional recurrence rate was significantly lower in the EPD group than in the SPD group (16.48% vs. 35.20%; P < 0.001; Supplementary Table S15). No difference was found in the total systemic recurrence rate between the two groups. However, the recurrence rate of mesenteric LNs was significantly higher in the SPD group (10.06% vs. 3.98%; P = 0.022; Supplementary Table S15), while peritoneal seeding was more frequently detected in the EPD group (17.61% vs. 8.94%; P = 0.029; Supplementary Table S15).
Concerning the pain intensity analysis between the EPD and SPD groups, baseline pain intensity was comparable, and no significant difference was found in changes in either upper abdominal pain or back pain intensity at 1 month postoperation from baseline between the two groups (ITT population, Supplementary Table S16). Similarly, no significant differences were found in changes in upper abdominal pain intensity at 6 months postoperation from baseline between the two groups (ITT population, Supplementary Table S17). In particular, at 6 months postoperation, in the EPD group, back pain intensity decreased from 1.02 (SD, 1.83) at baseline to 0.71 (SD, 1.51), while in the SPD group, back pain intensity increased from 1.07 (SD, 1.73) at baseline to 1.43 (SD, 2.42), with a significant change of -0.82 (95% CI, -1.37 to -0.29; P = 0.003; Supplementary Table S17).
4 DISCUSSION
For many years, the delicate surgical procedures for pancreatic head cancer have not been unified, and the range of surgical resection (including the LNs and nerves), margin of resection and usefulness of combined vascular resection remain controversial. An updated meta-analysis from Wang et al. [19], which included 8 studies involving 687 (342 vs. 345) patients, showed that radical dissection failed to improve OS and may have even led to increased morbidity. Concerning the overall data analysis, our study was similar to previous RCTs, and we concluded that extended dissection did not benefit all patients with potentially curable pancreatic head cancer. Nevertheless, the secondary endpoint DFS was superior to EPD. As safe and reliable as SPD, EPD led to a significant improvement in DFS for patients with pancreatic head cancer. Specifically, EPD significantly increased both OS and DFS in patients with a low chance (preoperative CA19–9 < 200.0 U/mL) of systemic metastasis in pancreatic head cancer. Our study provides high-level evidence for a significant benefit from EPD in patients with preoperative CA19–9 < 200.0 U/mL. Together with the predefined subgroup analysis of patients with a preoperative CA19–9 < 200.0 U/mL, this suggests a clinically relevant benefit of EPD in patients with pancreatic head cancer with a low probability of micrometastasis.
Regarding the morbidity of extended pancreatoduodenectomy, Wang et al. showed that the incidence of diarrhea (three months postoperatively) was significantly higher with EPD than with SPD [19]. This finding was attributed mainly to the circumferential dissection of the nerve plexus around the celiac axis and SMA in RCTs from the Mayo Clinic and Japan. In our study, we dissected the nerves and soft tissues at 270° on the right side surrounding the right longitudinal axis of the celiac-aorta-SMA artery axis. Our results showed that the rate of diarrhea was comparable between the SPD (5.03%) and EPD (7.46%) groups (Supplementary Table S3), similar to an RCT from Korea, in which the right half of the nerve plexus was dissected [2]. Retroperitoneal nerve dissection involves opening the arterial sheath. Although EPD tended to increase the rate of postoperative pseudoaneurysm (EPD vs. SPD, 1.99% vs.1.01%, Supplementary Table S3), there was no significant difference between the two groups. However, by routinely performing abdominal enhancement computed tomography angiography (CTA) within one week of surgery, we could effectively detect pseudoaneurysms and prevent life-threatening complications caused by the rupture of pseudoaneurysms through angiographic embolization or membrane stent implantation (only one patient died of severe sepsis with gastroduodenal artery rupture in the EPD group, and the proportions of aneurysm-related reoperations of EPD vs. SPD were 1.00% vs. 1.01% [Supplementary Table S3], with no significant difference).
Surgical techniques that have been developed to refine oncological resections and surgeons may be able to impact local control by the radicality and quality of surgical resection. Techniques used to achieve local radicality include artery-first approaches, the triangle operation, extended resections and level-3 dissection with the removal of the nerves and soft tissues surrounding the artery. To avoid complications from extended resections, we modified our procedure to refine the range to dissect the nerve and soft tissues at 270° around the right longitudinal axis of the celiac axis and SMA.
At present, evidence on the extent of nerve dissection for pancreatic head cancer remains insufficient. There are currently only 3 RCTs on the extent of nerve dissection for pancreatic head cancer. As early as the last decade, Japanese scholars emphasized that 360° circumferential dissection of the nerve plexus around the celiac axis and SMA improved R0 resection, but both RCTs from Japan reported that extended pancreatectomy led to intractable diarrhea, malnutrition and low quality of life, which in turn affected patient prognosis. Based on the modified extent of nerve plexus dissection in Japan, nerve plexus dissection with a right-sided range of 180° was utilized in the late-stage RCT performed by Korean scholars [2]. The results suggested no increase in the risk of intractable diarrhea leading to postoperative malnutrition and affecting the quality of life. Similar results were obtained from our trial, whereby we found that extended retroperitoneal LN dissection and right-sided 270° dissection of the nerve plexus around the celiac axis and SMA did not increase the morbidity or mortality rates. The rate of postoperative diarrhea at 3 months was similar between the SPD (5.03%) and EPD (7.46%) groups, with none of these patients experiencing severe intractable diarrhea.
Although the invasion of the extrapancreatic nerve in pancreatic cancer has been indicated as a poor prognostic factor in previous studies [7, 20, 21], the prognostic values of different levels of nerve plexus involvement remain unclear. In our study, we obtained the invasion status of different nerve plexuses in the EPD group. The univariate analysis of OS according to retroperitoneal plexus invasion showed that patients with positive invasion of the aortic plexus showed worse OS in EPD treatment (Supplementary Table S14), which suggested that this group of patients may need more individualized follow-up and adjuvant therapy after surgery. The perioperative CA19–9 level is one of the most reliable tumor markers for assessing pancreatic cancer. An elevated preoperative CA19–9 level is believed to be an independent predictor of early postoperative recurrence and metastasis, even if R0 surgical resection is achieved [9, 22]. Forsmark et al. reported that CA 19–9 levels greater than 300 U/mL indicated an advanced stage of pancreatic cancer and increased the risk of unresectability, but their small sample limited their research results [10]. Furthermore, multivariate regression analysis demonstrated that the independent contributing factor to resectability (R0 resection) was a preoperative CA 19–9 level < 92.77 U/mL [11]. Our results showed that the sufficient dissection of nerve tissues at the retroperitoneum and LNs around the pancreatic head significantly improved the prognosis of patients with pancreatic head cancer whose preoperative CA19–9 level was < 200.0 U/mL. The actual OS rate at 3 years was highly similar to the predicted rate and was approximately 15% higher in the EPD group than in the SPD group (40.19% vs. 26.47%, P = 0.041; Table 3). In line with our findings, an analysis from Japan showed that for pancreatic cancer patients with LN16 positivity, surgical resection and extended lymphadenectomy significantly improved the OS of those with a preoperative CA19–9 ≤ 360 U/mL compared with bypass surgery [23]. Kim et al. reported that markedly elevated preoperative CA19–9 levels might reflect unresectability in pancreatic adenocarcinoma patients who were thought to have resectable disease on preoperative imaging [11]. The PRODIGE 24 trial showed that the modified “fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX)” regimen led to significantly longer survival than gemcitabine among patients with resected pancreatic cancer. Meanwhile, to minimize the risk of the incorrect inclusion of patients with metastatic disease, only patients with postoperative serum CA19–9 levels < 180 U/mL were included [24]. In this study, according to the RCS model and ROC analysis, we recommend the cutoff point of CA19–9 as 200.0 U/mL for stratified treatment options in pancreatic head cancer.
In our trial, we found no prognostic difference between the two groups of patients with preoperative CA19–9 ≥ 200.0 U/mL, which indicates that micrometastasis might be the main contributor to prognosis. In our trial, when the rate of the recurrence pattern manifested only as systemic metastasis, the proportion was as high as 80.43% (148 out of 184) in the subgroup with preoperative CA19–9 ≥ 200.0 U/mL, which probably resulted in an underpowered study. The key point that might need to be discussed in this subgroup is upfront surgery or neoadjuvant chemoradiotherapy. A previous retrospective analysis showed that normalizing postoperative CA19–9 levels was a strong prognostic marker for long-term survival [25]. In regard to patients who had high preoperative CA19–9 ≥ 200.0 U/mL, we found that patients whose CA19–9 levels decreased to normal 2 weeks after surgery had a significantly better outcome than those with elevated CA19–9 levels (Supplementary Figure S3K-L), further emphasizing on the important predictive value of CA19–9 for prognosis. Nonetheless, patients with pancreatic head cancer are often associated with cholangitis, pancreatitis and obstructive jaundice, resulting in the elevation of CA19–9 [26, 27]. In our trial, patients with jaundice should be tested for baseline CA19–9 after adequate jaundice-reducing treatment and/or bile drainage.
Moreover, in the subgroup with well and moderate pathological differentiation (WD and MD, n = 224), the median OS (subgroup from the ITT population) in the EPD group was 6.2 months longer than that in the SPD group (P = 0.020, Supplementary Figure S2C). Correspondingly, the median DFS in the EPD group was 5.8 months longer than that in the SPD group (P = 0.006, Supplementary Figure S2D). Furthermore, in the EPD group, the subgroup with a preoperative CEA level less than 5.00 ng/mL (Supplementary Figure S2B), with a resectable stage (Supplementary Figure S3H), without PV/SMV resection (Supplementary Figure S2F) or who underwent R0 resection (Supplementary Figure S2H) and who received postoperative adjuvant chemotherapy (Supplementary Figure S2J) achieved better outcomes based on the DFS analysis (Supplementary Table S5). Interestingly, factors including postoperative CA19–9 < 200.0 U/mL, N0 stage, and well and moderate pathological differentiation significantly predicted a good prognosis in both the univariate and multivariate analyses (Supplementary Table S13). These results also strengthened our conclusion that EPD with retroperitoneal nerve resection in patients with pancreatic head cancer significantly improved oncological outcomes in those in an early stage with lower potential metastasis than SPD.
Approximately 20% of the patients in this study required vein reconstruction (Table 1), which means that this group was in the borderline stage and had a higher chance of micrometastasis. Our subgroup analysis confirmed that EPD did not show an advantage over SPD for patients with PV/SMV reconstruction (Supplementary Table S5), but for the subgroup who did not require vein reconstruction, we found a significant improvement in DFS in the EPD group (Supplementary Figure S2F). For patients requiring vascular resection, neoadjuvant chemotherapy should be given priority according to current guidelines. An interesting new topic is whether EPD combined with vascular resection is a better option after neoadjuvant therapy, which we will explore in the future. In the absence of RCTs to support the extent of dissection and the treatment sequencing strategy in subgroups of patients with resectable pancreatic head cancer, hopefully, our data will encourage more open trials to provide a more precise strategy for different subgroups of patients with resectable pancreatic head cancer.
In this study, most of the patients (343/400, 85.75%) accepted postoperative adjuvant chemotherapy, with a comparable rate in the SPD group and the EPD group (86.93% vs.84.58%, P = 0.568, Table 1) and the proportion of patients who received complete chemotherapy between the two groups was similar (EPD vs. SPD, 62.19% vs. 65.33%, P = 0.534, Table 1), which indicates that EPD did not significantly affect the postoperative chemotherapy acceptance rate of patients. The median OS in the adjuvant chemotherapy group (mainly gemcitabine single-agent chemotherapy) was similar to that reported in previous phase 3 trials of adjuvant therapy (24.4 months vs. 20.1 to 26.5 months), although the median DFS was slightly longer in our trial (17.7 months vs. 11.3 to 15.3 months) [28-32]. Of the 343 patients who underwent postoperative adjuvant chemotherapy, DFS was significantly longer in the EPD group than in the SPD group, although the difference was only 3.2 months (17.7 months vs. 14.5 months, P = 0.019, Supplementary Figure S2J). These results were inconsistent with those from a Korean study [2], in which adjuvant treatment had no effect on survival in the extended resection group. Vascular injury by extensive dissection was considered by a Korean study to reduce the postoperative effects of chemoradiation, whereas both our study and the Korean study showed that the major type of pancreatic cancer recurrence after surgery was systemic recurrence (locoregional vs. systemic for extended resection, 96.6% vs. 25.9% in the Korean study, 89.77% vs. 16.48% in our study, Supplementary Table S15), and the main effect of chemotherapeutics should be to preferentially reduce tumor spread or metastasis rather than locoregional recurrence.
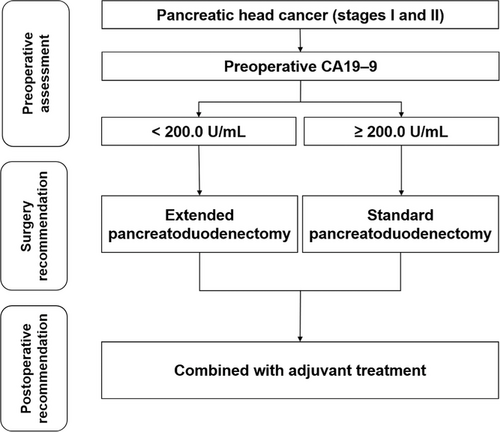
Furthermore, in the subgroup with CA19–9 < 200.0 U/mL, the benefit of EPD compared with SPD was the most significant in the subgroup that received chemotherapy (Supplementary Table S7), while for the subgroup with CA19–9 ≥ 200.0 U/mL, EPD showed no significant benefit in the population with and without adjuvant therapy (Supplementary Table S8). Based on this study, for pancreatic head cancer in stages I and II, we recommend EPD combined with adjuvant chemotherapy for patients with preoperative CA19–9 < 200.0 U/mL and SPD combined with adjuvant chemotherapy for patients with CA19–9 ≥ 200.0 U/mL (Figure 5)
Considering that a large proportion of PDACs deemed resectable by conventional imaging are likely metastatic [9], neoadjuvant treatment in high-risk PDAC patients may provide a chance for tumor downsizing or even downstaging, thereby improving surgical outcomes. Thus, the critical question raised by our trial is whether we can achieve a better outcome when extended surgery is performed for patients with high-risk micrometastasis after preoperative chemotherapy, which could indeed treat micrometastases and/or limit tumor spread during surgery [33]. Recent studies have suggested the possibility of using preoperative CA19–9 levels to discern patient subgroups that would benefit from upfront surgery or neoadjuvant therapy [34, 35]. Currently, neoadjuvant therapy is the standard strategy for patients with borderline pancreatic cancer. After neoadjuvant therapy to control or exclude micrometastases, it is more meaningful for patients to undergo EPD, which has more advantages in achieving adequate dissection of retroperitoneal nerve tissues and lymph nodes and R0 resection. Therefore, we believe it may be the best choice for patients to receive systemic treatment based on adequate radical surgery (EPD is more likely to achieve local radical resection than SPD). In addition, pancreatoduodenectomy surgery has entered the era of minimally invasive surgery, including laparoscopic and robotic pancreatoduodenectomy. At present, minimally invasive PD (MIPD) and open pancreatoduodenectomy (OPD) are basically the same in terms of the selection of patients, except for the unique contraindications of minimally invasive procedures such as the inability to tolerate pneumoperitoneum or the inability to safely establish pneumoperitoneum [36]. Several studies have shown that minimally invasive EPD with vascular replacement is safe and feasible with highly experienced surgeons and in high-volume pancreatic centers [37-39], although one study reported that a higher proportion of complex segmental resections was performed in the OPD group than in the LPD group [39]. Although the data are limited, we believe that the inherent advantages of minimally invasive approaches and delicate manipulation will lead to accurate results for the EPD of pancreatic head cancer. Our team is conducting RCTs to explore the short-term and long-term oncological effects of MIPD in treating pancreatic head cancer [40]. In the future, we will further explore the safety of minimally invasive EPD and which subgroups of patients can truly benefit from minimally invasive EPD.
In addition, our PSM analysis suggested that there were no significant confounding factors in this study, which further affirmed the validity of the randomization and the reliability of the results of this study. Furthermore, we supplemented the table with data on loss to follow-up, which was only 2.25% (Supplementary Table S18). The table shows that the missing data were mainly within 1 year of follow-up, and the time period of loss to follow-up in the two groups was very similar. The Gehan-Breslow-Wilcoxon test analysis was used to extra weight for early time points, and the results showed that the missing data did not significantly impact the results in the ITT population (Supplementary Table S19) or in subpopulation based on a predefined preoperative CA19–9 level [< 200.0 U/mL or ≥ 200.0 U/mL] (Supplementary Table S20), further indicating the reliability of the study.
The goal of a cure for patients with resectable pancreatic cancer is careful management of the surgical margin to ensure adequate tumor clearance. Although there was no difference in the total recurrence rate between the two groups, the locoregional recurrence rate was significantly lower in the EPD group than in the SPD group (EPD vs. SPD, 16.48% vs. 35.20%, P < 0.001, Supplementary Table S15). Moreover, the recurrence rate of mesenteric LNs was significantly higher in the SPD group (SPD vs. EPD, 10.06% vs. 3.98%, P = 0.022, Supplementary Table S15), while peritoneal seeding was more frequently detected in the EPD group (EPD vs. SPD, 17.61% vs. 8.94%, P = 0.029, Supplementary Table S15), which may have been caused by prolonged and extended manipulation around the tumor.
Nevertheless, this study has limitations. First, the patients enrolled in this study included patients with borderline resectable disease who had not been screened for neoadjuvant therapy and were at high risk of micrometastasis. Second, the postoperative chemotherapy regimen used in this study was gemcitabine as a single agent, which may be less effective in controlling systemic recurrence than albumin-bound paclitaxel plus gemcitabine (AG)and FOLFININOX regimens. Intolerance after chemotherapy in some patients and changes in the regimen after recurrence may have also caused a bias in survival prognosis between the two groups. Third, the subgroup analysis was stratified based on a predefined preoperative CA19–9 level [< 200.0 U/mL or ≥ 200.0 U/mL], and there was no prior detection of Lewis negative status. Approximately 5 to 10% of the population who are negative for the Lewis antigen have no or scarce secretion of CA19–9, causing false negative results. A previous study reported that among Lewis antigen-negative individuals with pancreatic cancer, high levels of CEA and CA125 were associated with a high risk of micrometastasis [41] and that the effect of radical surgery was attenuated in this subgroup of patients. Due to the limited number of patients, we did not perform further stratified analyses of these confounding factors in our study.
In this multicenter RCT comparing EPD and SPD for pancreatic head cancer, the difference in OS was not statistically significant, but we observed that the patients in the EPD arm had significantly improved DFS. In the subgroup analysis, improvements in both OS and DFS in the EPD arm were observed in patients with CA19–9 < 200.0 U/mL. Considering that there was no significant difference in mortality and morbidity between the two groups, EPD could be used as an effective surgical procedure for patients with pancreatic head cancer, especially those with preoperative CA19–9 < 200.0 U/mL.
DECLARATIONS
AUTHOR CONTRIBUTIONS
Rufu Chen: study concept and design; surgeon performing procedures; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtained funding; administrative, technical, or material support; study supervision; approval of the final version of the manuscript; informed consent and data verification. Qing Lin, Shangyou Zheng, Yu Zhou, Zhihua Li: acquisition of data; analysis and interpretation of data; statistical analysis; drafting of the manuscript, approval of the final version of the manuscript; informed consent and data verification. Xianjun Yu, Meifu Chen Yixiong Li, Weilin Wang, Renyi Qin: study concept and design; surgeon performing procedures; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; administrative, technical, or material support; study supervision; approval of the final version of the manuscript; informed consent and data verification. Quanbo Zhou, Chonghui Hu, Zhongdong Xu, Lin Wang, Yimin Liu, Min Wang, Guolin Li, He Cheng, Dongkai Zhou, Guodong Liu, Zhiqiang Fu, Yu Long: acquisition of data; analysis and interpretation of data; approval of the final version of the manuscript. Jing Gu: Analysis and interpretation of data; statistical analysis; approval of the final version of the manuscript. Qingyu Liu: Radiologist interpreting cross-sectional imaging in the trial; critical revision of the manuscript; approval of the final version of the manuscript. The corresponding authors are responsible for all aspects of this study, including the entire contents and all data.
ACKNOWLEDGMENTS
The authors would like to thank all patients who consented to be screened and who participated in the study. We are grateful to our colleagues and research staff who participated in the enrollment and evaluation of the patients at each center. We also thank Prof. Jie Wang and Prof. Quanxing Ni for advice on the study design; Prof. Chunyou Wang and Prof. Zhengxi Jin from the Pancreatic Cancer Committee of the Chinese Anti-Cancer Association as third-party experts for the independent evaluation to avoid intentional or unintentional patient enrollment; Prof. Yamei Tang for critical revision of the manuscript.
CONFLICT OF INTEREST DISCLOSURES
We declare no competing interests.
DATA SHARING STATEMENT
Individual de-identified participant data that underlie the results reported in this article and study protocol will be shared with investigators whose proposed use of the data has been approved by an independent review committee. Data can only be used to achieve aims in the approved proposal. Data will be available 6-36 months after the article is published. To gain access, data requesters will need to sign a data access agreement. Proposals should be directed to CRF ([email protected]).
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This trial was approved by all independent institutional review boards and ethics committees at each participating site. All study-related procedures were performed with the written informed consent of all patients. This study complies with the Declaration of Helsinki and the relevant provisions of the guidelines in both the collection of data and the use of materials.
CONSENT FOR PUBLICATION
Not applicable.




