Improved esophageal squamous cell carcinoma screening effectiveness by risk-stratified endoscopic screening: evidence from high-risk areas in China
Abstract
Background
Risk-stratified endoscopic screening (RSES), which offers endoscopy to those with a high risk of esophageal cancer, has the potential to increase effectiveness and reduce endoscopic demands compared with the universal screening strategy (i.e., endoscopic screening for all targets without risk prediction). Evidence of RSES in high-risk areas of China is limited. This study aimed to estimate whether RSES based on a 22-score esophageal squamous cell carcinoma (ESCC) risk prediction model could optimize the universal endoscopic screening strategy for ESCC screening in high-risk areas of China.
Methods
Eight epidemiological variables in the ESCC risk prediction model were collected retrospectively from 26,618 individuals aged 40-69 from three high-risk areas of China who underwent endoscopic screening between May 2015 and July 2017. The model's performance was estimated using the area under the curve (AUC). Participants were categorized into a high-risk group and a low-risk group with a cutoff score having sensitivities of both ESCC and severe dysplasia and above (SDA) at more than 90.0%.
Results
The ESCC risk prediction model had an AUC of 0.80 (95% confidence interval: 0.75–0.84) in this external population. We found that a score of 8 (ranging from 0 to 22) had a sensitivity of 94.2% for ESCC and 92.5% for SDA. The RSES strategy using this threshold score would allow 50.6% of endoscopies to be avoided and save approximately US$ 0.59 million compared to universal endoscopic screening among 26,618 participants. In addition, a higher prevalence of SDA (1.7% vs. 0.9%), a lower number need to screen (60 vs. 111), and a lower average cost per detected SDA (US$ 3.22 thousand vs. US$ 5.45 thousand) could have been obtained by the RSES strategy.
Conclusions
The RSES strategy based on individual risk has the potential to optimize the universal endoscopic screening strategy in ESCC high-risk areas of China.
Abbreviations
-
- AUC
-
- area under the receiver operating characteristic curve
-
- BMI
-
- body mass index
-
- ESCC
-
- esophageal squamous cell carcinoma
-
- H-L
-
- Hosmer-Lemeshow
-
- NPV
-
- negative predictive values
-
- NNS
-
- number needed to screen
-
- PPV
-
- positive predictive value
-
- RCT
-
- randomized controlled trial
-
- SDA
-
- severe esophageal dysplasia and above
-
- UGI
-
- upper gastrointestinal
1 BACKGROUND
Esophageal cancer (EC) remains the fourth leading cause of cancer death and the sixth most commonly diagnosed malignancy in China [1], with esophageal squamous cell carcinoma (ESCC) being the predominant histological type [2]. The prognosis of patients diagnosed with EC has remained poor in Chinese population, with a 5-year overall survival rate less than 40% [3]. Screening, early detection, and early treatment are widely accepted as effective strategies to reduce EC mortality [4, 5], and endoscopy with iodine staining has been the gold standard technique for the diagnosis of ESCC and its precursor lesions [6, 7]. Population-based EC screening trials have been conducted in high-risk areas of China for decades, providing endoscopic screening for all asymptomatic individuals aged 40-69 years (i.e., universal endoscopic screening) [8-13]. Reductions in EC mortality have been observed among participants screened with endoscopy compared to those not screened [8, 13]. A notable limitation of this universal endoscopic screening is the considerable costs associated with the endoscopic demands posed by millions of eligible individuals in high-risk areas of China [14, 15], which largely limits its implementation in China.
Several recent studies indicated that risk-stratified endoscopic screening (RSES), which offers no screening for those at lower risk while providing endoscopic screening for those with a higher risk of EC, could decrease endoscopic demands and improve screening effectiveness compared with the universal endoscopic screening strategy [16-20]. Risk prediction models based on easily available individualized risk factors for EC have shown promising usefulness for RSES in screening practice. Evidence of RSES in high-risk areas of China is limited to the best of our knowledge, and no available assessment tool for risk-stratified ESCC screening has been validated externally in the Chinese population. We previously developed a 22-score ESCC risk prediction model based on a multicenter prospective cohort in a Chinese population to identify those with a high risk of ESCC for whom endoscopy was recommended [21]. This model included eight epidemiological variables of age, sex, family history of upper gastrointestinal (UGI) cancer, smoking status, consumption of salted food, consumption of fresh fruits, nonspecific alarming symptoms of retrosternal pain, back pain, or neck pain, and disease history of peptic ulcer or esophagitis, with the total ESCC risk score ranging from 0 to 22. This model performed well with an area under the receiver operating characteristic curve (AUC) of 0.80 and provided a feasible tool for RSES; it could be hypothesized that RSES, in providing endoscopic screening only for high-risk individuals, would improve the screening performance of universal endoscopic strategy and decrease endoscopic demands for those with low risk of ESCC.
Therefore, in the present analysis, we aimed to assess the screening performance of the 22-score ESCC prediction model in a more representative population who underwent endoscopic screening in three high-risk areas of China as part of the screening arm of a multicenter randomized controlled trial (RCT) for UGI cancer screening. We further estimated whether the RSES strategy based on this model could optimize the universal endoscopic screening in high-risk areas of China by comparing their diagnostic yields and costs in one-off endoscopic screening.
2 METHODS
2.1 Study design and study population
We performed a cross-sectional analysis among a general population from three UGI cancer high-risk areas of China (Linzhou County of Henan Province, Cixian County of Hebei Province, and Wuwei County of Gansu Province) who underwent endoscopic screening between May 2015 and July 2017. The definition of high-risk areas in China was generated from the First National Death Survey in 1973-1975 [22]. High-risk areas for EC (such as Linzhou and Cixian) were defined as those with a crude mortality rate from EC of more than 60/100,000 in males and 30/100,000 in females. High-risk areas for gastric cancer (such as Wuwei) were defined as those with a crude mortality rate from gastric cancer of more than 50/100,000 in males and 25/100,000 in females. Detailed designs and methods on this RCT have been published previously [10, 23]. The trial was registered with the Protocol Registration System in the Chinese Clinical Trial Registry (identifier: ChiCTR-EOR-16008577) and approved by the independent ethics committee of the National Cancer Center (NCC) of China/Cancer Hospital, Chinese Academy of Medical Sciences (2015SQ00223). Each participant involved in this program provided informed consent.
In the aforementioned three high-risk areas, a total of 163 villages that met the criteria for cluster inclusion were included in the trial; these villages constituted the randomization units [23]. Clusters that had implemented endoscopic screening in the late 3 years and those that were unwilling to participate were excluded. The remaining clusters were randomly allocated to the screening arm or control arm at a ratio of 1:1 (81 villages in the screening arm and 82 villages in the control arm) using a stratified cluster sampling design, which has been described in detail previously [23]. The present study included participants from the 81 villages in the screening arm of the three high-risk areas, who were used for external validation of the developed model. This trial was open to all local residents aged 40-69 years with the ability to participate. The exclusion criteria were as follows: (1) participants who were diagnosed with cancer before recruitment or (2) participants who had an endoscopic examination in the last three years. Based on this trial, the present study further excluded participants who had incomplete information from the developed model.
2.2 Data collection
All eligible participants in the screening arm were assigned to undergo standard UGI endoscopic examination and biopsy with iodine staining. The entire esophagus and stomach were visually examined, and biopsies were taken from suspicious lesions. Biopsies were fixed in 10-13% formaldehyde, embedded in paraffin, and stained with hematoxylin and eosin. Two experienced pathologists independently reviewed the biopsy slides, and diagnostic discrepancies were adjudicated by consultation. Approximately 1% of the slides were randomly selected and blindly reviewed by a senior external pathologist from the NCC of China, and the consensus was higher than 98.0%. Histological criteria were as previously described [6, 23]. Severe esophageal dysplasia and above (SDA) lesions, which included severe esophageal dysplasia, squamous carcinoma in situ, and ESCC, were classified as positive cases for esophageal endoscopic screening.
Before endoscopic examinations, all recruited participants were interviewed by trained staff to complete a baseline, computer-aided, one-on-one questionnaire to gather information regarding their exposure to potential risk factors [10]. The baseline questionnaire covered variables in the ESCC risk scoring system developed by Chen et al. [21], except for one subitem of unexplained neck pain which was included in the variable of nonspecific alarming symptoms. Cumulative scores ranging from 0 to 22 were derived for each participant using the scoring rule by Chen et al. [21].
2.3 Outcome measures
In the present study, the primary outcome was ESCC, which is consistent with the previously developed prediction model. The second outcome was SDA, which is an important index for EC screening.
2.4 Statistical analysis
The AUC, Hosmer-Lemeshow test (goodness-of-fit test), and calibration curves were used to evaluate the model's performance.
We calculated the sensitivities of endoscopy for the high-risk population based on the 22-score ESCC risk prediction model. For the RSES strategy, we set the cutoff value as the maximum score in the model having both ESCC and SDA sensitivities of more than 90.0%. The population was then stratified into low-risk and high-risk groups. Next, we analyzed and estimated the diagnostic performance of the universal endoscopic screening strategy versus that of the RSES strategy based on the outcomes of ESCC and SDA lesions. The estimated indices included the number and proportion of high-risk individuals, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and number needed to screen (NNS) to detect one case via endoscopy. Sensitivity was calculated as the proportion of true cases identified by the prediction model among all true cases diagnosed by pathological diagnosis. Specificity was calculated as the proportion of true non-cases identified by the prediction model among all non-cases diagnosed by pathological diagnosis. PPV was calculated as the proportion of true cases identified by the prediction model among the high-risk population identified by the prediction model. NPV was calculated as the proportion of true negative cases identified by the prediction model among the low-risk population identified by the prediction model. NNS was calculated as the proportion of the high-risk population identified by the prediction model among the true cases identified by the prediction model.
2.5 Cost considerations
The cost analysis focused on a cost comparison of the two screening strategies in one-off endoscopic screening rather than lifetime cost-effectiveness. The total costs were approximately calculated for the following three aspects: screening mobilization and administration (US$ 1.05/person), epidemiological survey and risk assessment (only for RSES; US$ 2.00/person), and clinical examination (US$ 47.87/person). The cost data were obtained from our previous survey of seven study centers from the aforementioned RCT (Supplementary Table S1). The costs of detecting one case of ESCC and SDA with the different strategies were calculated using the following equations: Cost per detected ESCC or SDA = total cost / total number of ESCC or SDA cases.
Analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA).
3 RESULTS
3.1 Participant characteristics
A total of 27,957 participants from 81 villages in the three aforementioned high-risk areas were recruited as the baseline. The percentage of participants who finished the questionnaire and endoscopic examination was 43.7% among those invited in the trial. A total of 1339 participants were excluded, with the remaining 26,618 eligible individuals included in the final analysis (Figure 1). Individuals from Cixian County, Linzhou County, and Wuwei County accounted for 26.0%, 36.7%, and 37.3% of the total, respectively. With the universal endoscopic screening strategy, 239 SDA lesions (including 52 ESCC cases) were identified, with SDA and ESCC detection rates of 0.9% and 0.2%, respectively.
Table 1 shows the major sociodemographic characteristics of the 26,618 participants from the three areas. Overall, the study population contained fewer males (43.1%) than females (56.9%) and fewer individuals aged 60s (25.3%) than those aged 40s (36.9%) and 50s (37.8%). Most of the study population were Han Chinese (99.8%) and married (94.0%). Education levels were relatively low, with 82.1% having only a primary or middle school degree and 16.7% reporting no schooling education. Table 2 shows the distribution of each risk factor of the developed ESCC prediction model in our study population. The study participants in our external validation were similar in age and sex distributions to those in the model development cohorts [21], but exhibited a higher prevalence of the other six risk factors.
| Characteristic | Total [n (%)] | Cixian County [n (%)] | Linzhou County [n (%)] | Wuwei County [n (%)] |
|---|---|---|---|---|
| Total | 26,618 (100.0) | 6910 (26.0) | 9766 (36.7) | 9942 (37.3) |
| Sex | ||||
| Men | 11,463 (43.1) | 2939 (42.5) | 3917 (40.1) | 4607 (46.3) |
| Women | 15,155 (56.9) | 3971 (57.5) | 5849 (59.9) | 5335 (53.7) |
| Age (years) | ||||
| 40-49 | 9824 (36.9) | 2211 (32.0) | 3312 (33.9) | 4301 (43.2) |
| 50-59 | 10,053 (37.8) | 2856 (41.3) | 3610 (37.0) | 3587 (36.1) |
| 60-69 | 6741 (25.3) | 1843 (26.7) | 2844 (29.1) | 2054 (20.7) |
| Ethnicity | ||||
| Han | 26,578 (99.8) | 6905 (99.9) | 9760 (99.9) | 9913 (99.7) |
| Others | 40 (0.2) | 5 (0.1) | 6 (0.1) | 29 (0.3) |
| Education† | ||||
| No schooling | 4443 (16.7) | 1437 (20.8) | 300 (3.1) | 2706 (27.2) |
| Primary or Middle school | 21,845 (82.1) | 5440 (78.8) | 9311 (95.3) | 7094 (71.4) |
| Senior high school and above | 328 (1.2) | 31 (0.4) | 155 (1.6) | 142 (1.4) |
| Marriage status‡ | ||||
| Unmarried | 165 (0.6) | 45 (0.7) | 49 (0.5) | 71 (0.7) |
| Married | 25,026 (94.0) | 6469 (93.7) | 9229 (94.5) | 9328 (93.8) |
| Divorced | 120 (0.5) | 34 (0.5) | 48 (0.5) | 38 (0.4) |
| Widowed | 1303 (4.9) | 360 (5.2) | 440 (4.5) | 503 (5.1) |
- † There are two missing values in Cixian County.
- ‡ There are four missing values, with two in Cixian County and two in Wuwei County.
| Predictor | Score | Total [n (%)] |
|---|---|---|
| Age (years) | ||
| 40-44 | 0 | 3765 (14.1) |
| 45-49 | 2.5 | 6059 (22.8) |
| 50-54 | 5.0 | 5839 (21.9) |
| 55-59 | 7.5 | 4214 (15.8) |
| 60-64 | 9.0 | 4275 (16.1) |
| 65-69 | 9.5 | 2466 (9.3) |
| Sex | ||
| Women | 0 | 15,155 (56.9) |
| Men | 1.5 | 11,463 (43.1) |
| Family history of UGI cancer | ||
| No | 0 | 19,955 (75.0) |
| Yes | 2.5 | 6663 (25.0) |
| Cigarette smoking (pack-years) | ||
| No | 0 | 19,713 (74.1) |
| <30 | 1.5 | 4669 (17.5) |
| ≥30 | 2.5 | 2236 (8.4) |
| Salted food consumption | ||
| Low | 0 | 25,017 (94.0) |
| High | 1.5 | 1601 (6.0) |
| Fresh fruit consumption | ||
| High | 0 | 14,405 (54.1) |
| Low | 1.0 | 12,213 (45.9) |
| Alarming symptoms† | ||
| No | 0 | 26,475 (99.5) |
| Yes | 2.0 | 143 (0.5) |
| Relative disease history | ||
| No | 0 | 25,613 (96.2) |
| Yes | 1.5 | 1005 (3.8) |
- Abbreviation: ESCC, esophageal squamous cell carcinoma; UGI, upper gastrointestinal.
- † Alarming symptoms include any current symptoms of retrosternal pain, back pain, or neck pain in the present study.
3.2 External validation of the ESCC prediction model
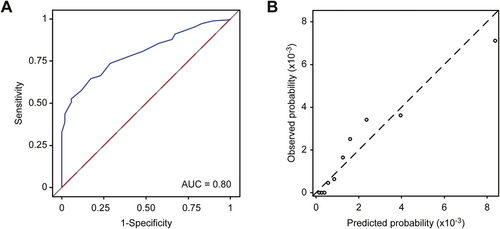
Despite the differences in the population characteristics, when we applied the risk scoring system to the external validation population, the discrimination of ESCC cases was good, with an AUC of 0.80 (95% confidence interval [CI]: 0.75-0.84; Figure 2A). The model demonstrated good calibration based on the goodness-of-fit test (χ2 = 9.43, P = 0.307) and the calibration curve (Figure 2B). Among the three independent areas, the AUCs varied from 0.73 in Cixian County to 0.86 in Wuwei County, but all the areas, the model had a good calibration ability based on the goodness-of-fit tests (Supplementary Table S2). The AUC for identifying SDA lesions was slightly lower at 0.78 (Supplementary Figure S1).
3.3 Performance of RSES versus universal endoscopic screening in high-risk areas of China
Table 3 and Supplementary Table S3 show the performance of universal endoscopic screening and various RSES strategies under different score-based cutoffs for ESCC and SDA, respectively. As the score increased, the sensitivities of ESCC and SDA both decreased, whereas the specificities increased. The maximum score with the sensitivity of both ESCC and SDA set to higher than 90.0% was 8, which thus served as the cutoff value. Populations with scores <8 and ≥8 were divided into a low-risk group and a high-risk group, respectively.
| Screening performance for ESCC | Screening cost (US$, thousand) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Screening strategy | Total [n (%)] | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | NNS | Total cost | Cost per detected ESCC | |
| Universal endoscopy | 26,618 (100.0) | 100.0 | 0.0 | 0.2 | NA | 512 | 1302.15 | 25.04 | |
| Risk-stratified endoscopy | Score 0 | 26,618 (100.0) | 100.0 | 0.0 | 0.2 | NA | 512 | 1355.39 | 26.07 |
| Score 1 | 25,814 (97.0) | 100.0 | 3.0 | 0.2 | 100.0 | 496 | 1316.90 | 25.33 | |
| Score 2 | 24,860 (93.4) | 100.0 | 6.6 | 0.2 | 100.0 | 478 | 1271.23 | 24.45 | |
| Score 3 | 23,196 (87.1) | 100.0 | 12.9 | 0.2 | 100.0 | 446 | 1191.58 | 22.91 | |
| Score 4 | 21,419 (80.5) | 100.0 | 19.6 | 0.2 | 100.0 | 412 | 1106.51 | 21.28 | |
| Score 5 | 20,541 (77.2) | 100.0 | 22.9 | 0.3 | 100.0 | 395 | 1064.48 | 20.47 | |
| Score 6 | 17,903 (67.3) | 100.0 | 32.8 | 0.3 | 100.0 | 344 | 938.20 | 18.04 | |
| Score 7 | 15,112 (56.8) | 98.1 | 43.3 | 0.3 | 100.0 | 296 | 804.60 | 15.78 | |
| Score 8 | 13,155 (49.4) | 94.2 | 50.7 | 0.4 | 100.0 | 268 | 710.91 | 14.51 | |
| Score 9 | 11,337 (42.6) | 88.5 | 57.5 | 0.4 | 100.0 | 246 | 623.89 | 13.56 | |
| Score 10 | 8950 (33.6) | 76.9 | 66.5 | 0.4 | 99.9 | 224 | 509.62 | 12.74 | |
| Score 11 | 5696 (21.4) | 57.7 | 78.7 | 0.5 | 99.9 | 190 | 353.85 | 11.80 | |
| Score 12 | 3843 (14.4) | 42.3 | 85.6 | 0.6 | 99.9 | 175 | 265.15 | 12.05 | |
| Score 13 | 2390 (9.0) | 32.7 | 91.1 | 0.7 | 99.9 | 141 | 195.59 | 11.51 | |
| Score 14 | 1276 (4.8) | 21.2 | 95.2 | 0.9 | 99.8 | 116 | 142.27 | 12.93 | |
| Score 15 | 493 (1.9) | 13.5 | 98.2 | 1.4 | 99.8 | 70 | 104.78 | 14.97 | |
| Score 16 | 255 (1.0) | 9.6 | 99.1 | 2.0 | 99.8 | 51 | 93.39 | 19.00 | |
- Abbreviations: ESCC, esophageal squamous cell carcinoma; PPV, positive predictive value; NPV, negative predictive value; NNS, number needed to screen to detect one ESCC case via endoscopy.
- Note: There were no ESCC cases when the score was 17 and above; therefore, we did not display the corresponding statistics of these cutoff values.
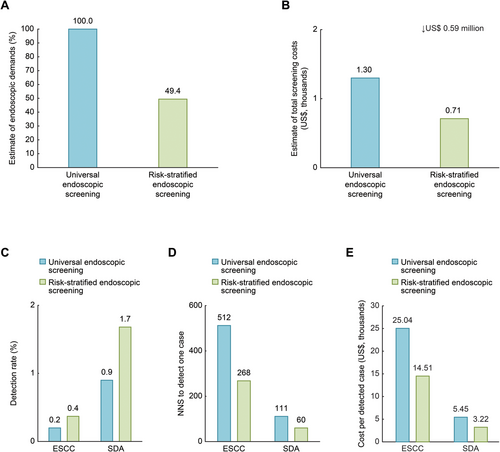
Figure 3 depicts the cost that could have been saved and screening performance that could have been obtained if this RSES strategy, where endoscopy is restricted to participants in high-risk populations, was implemented among the 26,618 participants between 2015 and 2017. Demand for endoscopy would decrease by 50.6% and save approximately 45.4% of the total screening costs (US$ 0.71 million vs. US$ 1.30 million) as compared with the universal endoscopic screening. The detection rates of ESCC would increase from 0.2% to 0.4%, and the detection rates of SDA lesions would increase from 0.9% to 1.7%. The NNS by endoscopy would decrease from 512 to 268 to identify one ESCC lesion and decrease from 111 to 60 to identify one SDA lesion. This RSES strategy could also save 42.1% of the average cost per detected ESCC (US$ 14.51 thousand vs. US$ 25.04 thousand) and save 40.9% of the average cost per detected SDA (US$ 3.22 thousand vs. US$ 5.45 thousand) compared with the universal endoscopic screening strategy. Further, this RSES strategy could help increase the screening efficiency for SDA in each high-risk area in our study population (Supplementary Tables S4–S6).
4 DISCUSSION
In this study, we estimated the screening performance of the RSES strategy based on a 22-score risk prediction model and the universal endoscopic screening strategy in identifying ESCC and SDA using population-based, cross-sectional, endoscopic screening data in three high-risk areas of China. Our findings showed that the 22-score ESCC prediction model performed well in identifying ESCC cases in high-risk areas of China. Compared to the universal endoscopic screening strategy, the RSES strategy could improve screening effectiveness and efficiency with increased detection rates of ESCC and SDA, decreased NNS to detect ESCC and SDA via endoscopy, and decreased cost per detected case of ESCC and SDA.
Several prediction models based on epidemiological variables have been proposed to identify high-risk populations for ESCC screening [17, 21, 24-26]. Our model showed good discrimination in the independent external population, with an AUC of 0.80 (95% CI: 0.75–0.84) and good calibration (χ2 = 9.43, P = 0.307 on the Hosmer-Lemeshow test), suggesting the applicability of this model. Note that we did not validate the other ESCC model developed by Wang et al. [16] or the SDA model developed by Liu et al. [17] due to the lack of corresponding predictive variables, such as the duration of living with a partner, pesticide exposure, regularity of eating, eating speed, and ingestion of leftover food during summer months.
The findings that demonstrated that RSES was superior to universal endoscopic screening were in line with previous observational studies [16-18]. Of note, there were many selections of risk cutoff values to optimize the universal endoscopy screening strategy in high-risk areas of China. One reasonable approach is to choose cutoff values to identify a subgroup population with high risk while retaining high sensitivities for both ESCC and SDA, as the sensitivities of ESCC and SDA were 100.0% under the universal strategy. Accordingly, the cutoff value of 8 in the score-based model meets this requirement, with sensitivities for ESCC and SDA remaining at 94.2% and 92.5%, respectively.
We found that this RSES strategy yielded an approximately 88.9% higher detection rate of ESCC and SDA and saved an approximately one half of NNS than the universal screening strategy, which indicated that the efficiency of endoscopic screening in high-risk areas of China would be doubled. In addition, the RSES strategy would allow approximately 50.6% of participants at low risk to avoid unnecessary endoscopic screening, which would save US$ 0.59 million in costs among the 26,618 participants and decrease the average cost per detected SDA by 40.9%. These findings preliminarily hint that a higher cost effectiveness could be obtained from the ESES strategy than from the universal endoscopic screening strategy in our study population from the three high-risk areas of China.
Although great benefits could be obtained by this RSES strategy and this strategy could be more suitable for resource-limited situations in high-risk areas in China, we must convey to policymakers that this RSES strategy would miss 7.5% of patients with SDA lesions. Actually, the selection of a threshold needs to be carefully determined by considering many practical factors and needs [20], and it is unlikely to be the same for different programs or trials. In the present study, we also found that the screening performance varied slightly across different study centers even though the same score-based cutoff values were applied (Supplementary Tables S4–S6). These findings suggest that additional considerations need to be taken when using the scoring system provided by this study and that reasonable cutoff values should be determined based on practical situations. We only performed a primary exploration of risk thresholds in high-risk areas of China based on our study population, and detecting optimal risk thresholds, especially for regions with different levels of disease burden and economic features, was beyond the scope of this study and needs to be addressed in future studies.
Our study has several unique strengths. First, this study estimated the validity and utilization of a proposed ESCC risk scoring system in a population undergoing mass EC screening in high-risk areas of China and considered the screening efficiency (i.e., the detection rate), effectiveness (i.e., the NNS), and cost (i.e., screening cost and the average cost per detected SDA). Second, this study was conducted based on a multicenter RCT for UGI cancer, which produced highly representative estimation data for the distribution of ESCC and SDA (i.e., positive cases according to endoscopic screening) in the general population. These data enabled us to accurately estimate the efficiency and effectiveness of different risk scores as thresholds to optimize the universal endoscopic screening strategy in high-risk areas of China.
Our study also had several limitations. First, this study used a cross-sectional design; therefore, the risk, i.e., the agreement between the predicted and actually observed risks, could not be calibrated. The participants in this study were limited to three high-risk areas in China, and more adequate estimations and validations in other high-risk areas need to be conducted in the future. In addition, some well-known important risk factors for ESCC, such as alcohol drinking or alcohol metabolic gene SNPs, were not covered in the risk scoring system. Therefore, the practical utility of the risk scoring system to places out of the high-risk areas in China and other countries is unknown, although this was not the scope of this study. The control arm of the RCT of UGI cancer screening, which covers rural, urban, high-risk, and non-high-risk areas of China, will provide high-quality and abundant data to fully validate and estimate the proposed ESCC risk scoring system when additional follow-up data are available in the future. Second, our study did not include a health economic evaluation, such as cost-effectiveness, when selecting the optimal score threshold, although we performed a preliminary economic exploration by estimating indices for the SDA detection rate, NNS, and approximate costs of detecting one case for each score threshold. We will conduct a detailed cost-effectiveness analysis to provide a comprehensive recommendation by considering the disease burden of EC and economic levels.
5 CONCLUSIONS
Among the population aged 40-69 years in three high-risk areas of China, endoscopies for the high-risk participants could achieve improved screening effectiveness and reduced screening costs than the universal endoscopic screening strategy can in one-off endoscopic screening. These findings support that RSES based on the ESCC risk prediction has the potential to optimize the ESCC screening strategy in high-risk areas of China. Future studies to validate the performance of the risk prediction model in other high-risk areas of China and in diverse populations, as well as to conduct cost-effect analyses to estimate the optimal threshold and feasibility, are warranted.
ACKNOWLEDGMENTS
We gratefully acknowledge all the participants in this trial and all of the staff who contributed to the data collection, auditing, database management, and verification. We thank all of the individuals who contributed to this study for their important and appreciated contributions to the preparation of this report.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The research protocols were independently approved by the Institutional Review Board of the Cancer Institute/Hospital, Chinese Academy of Medical Sciences (2015SQ00223). Each participant involved in this program signed an informed consent form.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest or financial interests to disclose.
FUNDING SUPPORT AND ROLE OF THE FUNDING SOURCE
This work was supported by the National Key R&D Program of China (2018YFC1313100), the Special Project of Beijing-Tianjin-Hebei Basic Research Cooperation (J200017), the Sanming Project of Medicine in Shenzhen (SZSM201911015), the Special Fund for Health Research in the Public Interest (201502001), and the Major State Basic Innovation Program of the Chinese Academy of Medical Sciences (2016-12 M-2-004 and 2019-I2 M-2-004). The funding source of the study had no role in the study design, data collection, analysis, or interpretation or writing of the report.
AUTHOR CONTRIBUTIONS
WC, JH, HL, and CD contributed to the study concept and design. HZ, RZ, HL, MC, DS, and SH contributed to the data quality. ZZ, XS, GG, and GS contributed to the data collection, data transmission and data correction after quality control. HL and DC drafted and finalized the paper with JR, JS, DS, ZY, YY and WC. All authors contributed to the data interpretation and manuscript revision. WC and JH had access to all of the raw data, and WC obtained the funding mentioned in the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
Additional summary tables, sensitivity analyses and data used in this research are available upon reasonable request from the corresponding author.




