Activity and bioavailability of tepotinib for leptomeningeal metastasis of NSCLC with MET exon 14 skipping mutation
Abstract
Tepotinib is a key drug for cancer patients with mesenchymal-epithelial transition receptor tyrosine kinase proto-oncogene (MET) exon 14 skipping mutation. However, its bioavailability in the cerebrospinal fluid (CSF) in humans has not been fully elucidated. Moreover, information about the efficacy of tepotinib in patients with leptomeningeal metastasis is limited. Here, we present the case of a 56-year-old man who was diagnosed with lung adenocarcinoma with MET exon 14 skipping mutation. He was urgently hospitalized due to leptomeningeal metastasis. We administered tepotinib 500 mg/day as the second-line therapy and observed improvement in leptomeningeal metastasis and performance status. The tepotinib concentrations reached 1,648 ng/mL in the plasma and 30.6 ng/mL in the CSF, with a penetration rate (CSF/plasma) of 1.83%. These demonstrate tepotinib could achieve a high rate of central nervous system transition and could be effective against leptomeningeal metastasis.
Abbreviations
-
- ALK
-
- anaplastic lymphoma kinase
-
- CSF
-
- cerebrospinal fluid
-
- CT
-
- computed tomography
-
- EGFR
-
- epidermal growth factor receptor
-
- GCS
-
- Glasgow Coma Scale
-
- IC50
-
- half-maximal inhibitory concentration
-
- LC-SCRUM
-
- Lung Cancer Genomic Screening Project for Individualized Medicine
-
- MET
-
- mesenchymal-epithelial transition receptor tyrosine kinase proto-oncogene
-
- MRI
-
- magnetic resonance imaging
-
- NSCLC
-
- non-small cell lung carcinoma
-
- PS
-
- performance status
-
- TKI
-
- tyrosine kinase inhibitor
INTRODUCTION
Lung cancer is a common cause of death worldwide [1]. Leptomeningeal carcinomatosis is a severe complication of lung cancer, and despite this condition is associated with short survival and poor performance status (PS) [2], patients with leptomeningeal carcinomatosis or poor PS are usually excluded from clinical trials. Some prospective phase II studies showed that molecular-targeted drugs such as epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI) [3] and anaplastic lymphoma kinase (ALK)-TKI [4] had a durable antitumor effect and could improve PS. Recently, tepotinib, a mesenchymal-epithelial transition receptor tyrosine kinase proto-oncogene (MET) inhibitor, was found to have durable clinical activity in non-small cell lung carcinoma (NSCLC) patients with MET exon 14 skipping [5]. Further, capmatinib, a highly-selective inhibitor of MET, also demonstrated promising antitumor activity in NSCLC patients with MET exon 14 skipping mutation [6]. These two MET inhibitors for NSCLC patients with MET exon 14 skipping mutation at the same time was approved. However, the effects of these molecular-targeted drugs on brain lesions have not yet been elucidated. Although previous studies have reported the physicochemical and pharmacological characteristics of EGFR-TKI and ALK-TKI in the central nervous system [7, 8], the efficacy and bioavailability of tepotinib in lung cancer patients with leptomeningeal metastasis remain unclear. Herein, we report a case of leptomeningeal metastasis successfully treated with tepotinib and the bioavailability of tepotinib in the cerebrospinal fluid (CSF) in a human case.
CASE REPORT
A 56-year-old man was referred to our hospital (Hirosaki University hospital, Japan) in August 2019 because of an abnormal shadow in the right upper lung on chest X-ray. The patient had a smoking history of 50 pack-years. However, his medical history was unremarkable and he did not take any medications. A transbronchial lung biopsy of the main lesion in the right upper lung was performed. The pathological diagnosis was adenocarcinoma of the lung (cT3N2M0, stage IIIA based on the eighth edition of the TNM classification for lung cancer). EGFR mutation and ALK fusion gene were not detected. Based on the decision of the tumor board of Hirosaki University hospital, the patient underwent right lung pneumonectomy in September 2019. However, post-operation follow up in October 2019 using computed tomography (CT) found brain metastasis in his left cerebrum, for which he underwent stereotactic radiosurgery and was prescribed with four continuous cycles of cisplatin (75 mg/m2) and pemetrexed (500mg/m2), and maintenance therapy with pemetrexed (500 mg/m2) as the first-line chemotherapy from November 2019 to April 2020. Treatment process from diagnosis to the last date of follow-up (October 29, 2020) is depicted in Figure 1.
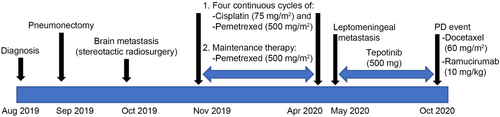
In May 2020, he was urgently hospitalized due to headache, vomiting, and decreased level of consciousness (Glasgow coma scale [GCS] score: E2, V2, M5). Brain magnetic resonance imaging (MRI) revealed linear high-intensity signals in the medulla, cerebellum, and cerebrum, indicating leptomeningeal metastasis and hydrocephalus (Figure 2A, B). The patient was diagnosed with leptomeningeal metastasis. Driver mutation was assessed using the OncomineTM Comprehensive Assay (Thermo Fisher Scientific, Waltham, Massachusetts, the USA) and AmoyDx Lung Cancer PCR Panel (9-in-1 assay) (Amoy Diagnostics, Xiamen, China). The results revealed MET exon 14 skipping mutation. c-MET gene amplification or other mutations were not detected. Gene screening was conducted in the Lung Cancer Genomic Screening Project for Individualized Medicine (LC-SCRUM) project in Japan. Although the patient's ECOG PS score was 4, tepotinib 500 mg once daily was initiated. Tepotinib was administered from May 31, 2020 to October 19, 2020. After 14 days of treatment, his PS score improved to 1, with a GCS score of E4, V5, M6. Brain MRI on June 13, 2020, revealed diminished leptomeningeal metastasis improved. (Fig. 2C, D). The adverse events were grade 2 edema and hypoalbuminemia, which were manageable. Five months after starting tepotinib treatment, disease progression was observed. New multiple liver metastasis appeared on CT in October, 2020 (Figure 2E) and third-line treatment with docetaxel and ramucirumab was started.
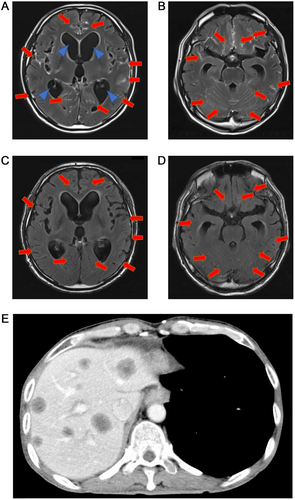
Follow-up imaging examinations of the cT3N2M0 lung adenocarcinoma case. (A-B) Follow-up brain MRI 9 months after post right lung pneumonectomy. Brain MRI showing high-intensity signals in the medulla cerebellum and cerebrum (long red arrows) as well as hydrocephalus (short blue arrows).(C-D) Brain MRI 14 days after initiation of tepotinib, showing the disappearance of multiple leptomeninges signals (long red arrows). (E) Computed tomography follow-up 5 month after the initiation of tepotinib. Multiple liver metastases could be found on the patient's liver. Abbreviations: MRI, magnetic resonance imaging
We also analyzed the plasma and CSF concentrations of tepotinib on day 20 of the treatment. Briefly, the blood samples were centrifuged at 3,500 rpm for 10 min at 4°C, and the separated plasma was stored at −80°C until analysis. The plasma and CSF concentrations of tepotinib were measured via ultra-performance liquid chromatography using the ACQUITY UPLC System (Waters Corp., Milford, MA). We obtained blood samples at 0, 4, 8, 12, and 24 h after tepotinib administration (Figure 3). CSF sample was obtained at 4 h after tepotinib administration. The average tepotinib concentrations was 1,648 ng/mL in the plasma and 30.6 ng/mL (62 nM) in the CSF with a penetration rate (CSF / plasma) of 1.83%. The blood concentration of tepotinib maintained the effective blood concentration (390 ng/mL to 823 ng/mL) at any time, and its concentrations in the cerebrospinal fluid were greater than the half-maximal inhibitory concentration (IC50).
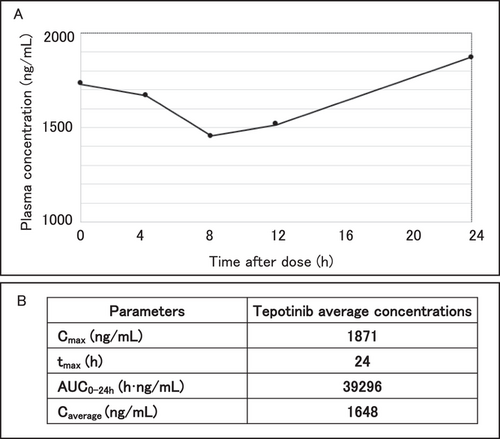
Pharmacokinetics of tepotinib at day 20 of the treatment. (A) Tepotinib concentrations in the patient's plasma after day 20 ranged from 1455 ng/mL to 1871 ng/mL. (B) Tepotinib average concentrations were 1648 ng/mL in plasma. Cmax and tmax were 1871 ng/mL and 24, respectively. Abbreviations: Cmax, maximum drug plasma concentration; tmax, maximum drug plasma concentration time; AUC0-24h, area under the drug plasma concentration-time curve (time 0 to 24h); Caverage, average drug plasma concentration
DISCUSSION
To the best of our knowledge, this is the first case report on leptomeningeal metastasis successfully treated with tepotinib in a patient with poor PS and the bioavailability of tepotinib in the CSF in human. Recently, the VISION trial showed that tepotinib had durable antitumor activity in patients with NSCLC with MET exon 14 skipping mutations. In Japan, ArcherMET (ArcherDX Inc., the USA) is used for companion diagnosis for MET exon 14 skipping mutations. In the VISION study, advanced NSCLC patients with asymptomatic brain metastases was enrolled [5]. The response rate of patients with brain metastases was 55%. In cohorts 4 and 5b of the A2201 phase II trial, 13 patients with brain metastases received capmatinib, and the response rate was 54% [6]. However, the cerebrospinal fluid transferability of capmatinib has not been elucidated. In a phase I study, Falchook et al. [9] reported that the tepotinib concentration in the plasma should range from 390 ng/mL to 823 ng/mL to achieve 95% phosphor-MET inhibition and an IC50 of 1.7 nM. In our case, tepotinib concentration in CSF was 30.6 ng/mL, which was enough to exceed the IC50. Moreover, Shitara and colleagues [10] conducted a phase I trial on Japanese patients, and observed an average steady-state concentration of tepotinib in the plasma, ranging from 760.3 ng/mL to 1126.8 ng/mL. Thus, the concentration of tepotinib in the CSF exceeded the IC50. Our patient had a higher average tepotinib concentration (1648 ng/mL) in plasma, which might accelerate drug transfer to the CSF. Penetration of the drug into the CSF is an important factor for central nervous system metastases. High-dose administration of EGFR-TKIs achieved high CSF concentrations of EGFR-TKIs [11].
In conclusion, tepotinib 500 mg/day demonstrated a high rate of central nervous system transition, and it demonstrated promising response for controlling leptomeningeal metastasis. Tepotinib could achieve high CSF concentration and this pinpoint the possible use of MET inhibitors in individual cases. However, further studies should be conducted to validate our findings.
DECLARATIONS
AUTHORSHIP
All authors have read and approved the final manuscript. All authors have contributed significantly and abided with the latest guidelines of the International Committee of Medical Journal Editors. HT prepared the manuscript; KT and TM treated and observed patients in Hirosaki University; JN and TN analyzed plasma and cerebrospinal fluid concentrations of tepotinib; ST reviewed the manuscript and advised radiological image findings.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Written informed consent was obtained from the patient.
CONSENT FOR PUBLICATION
Informed consent was obtained from the patient's family for publication and pharmacokinetics analysis of this case report and accompanying images.
CONFLICT OF INTEREST STATEMENT
All authors declare they have no conflicts of interest.
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
ACKNOWLEDGMENTS
We thank the patient and their families as well as Dr. Shingo Matsumoto of the National Cancer Center Hospital East for his expertise in next-generation sequences.
Open Research
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this case study are included in this published article.




