Molecular subtyping of hepatocellular carcinoma: A step toward precision medicine
Abstract
Hepatocellular carcinoma (HCC) is one of the most prevalent and fatal digestive tumors. Treatment for this disease has been constraint by heterogeneity of this group of tumors, which has greatly limited the progress in personalized therapy. Although existing studies have revealed the genetic and epigenetic blueprints that drive HCCs, many of the molecular mechanisms that lead to HCCs remain elusive. Recent advances in techniques for studying functional genomics, such as genome sequencing and transcriptomic analyses, have led to the discovery of molecular mechanisms that participate in the initiation and evolution of HCC. Integrative multi-omics analyses have identified several molecular subtypes of HCC associated with specific molecular characteristics and clinical outcomes. Deciphering similar molecular features among highly heterogeneous HCC patients is a prerequisite to implementation of personalized therapeutics. This review summarizes the current research progresses in precision therapy on the backbone of molecular subtypes of HCC.
Abbreviations
-
- AFP
-
- α-fetoprotein
-
- BCLC
-
- Barcelona Clinic Liver Cancer
-
- CTLA-4
-
- cytotoxic T-lymphocyte-associated protein 4
-
- CTNNB1
-
- catenin beta-1
-
- EPCAM
-
- epithelial cell adhesion molecule
-
- FGFR
-
- fibroblast growth factor receptor
-
- FLT3
-
- Fms-like tyrosine kinase 3
-
- HBV
-
- hepatitis B virus
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- HER2
-
- human epidermal growth factor receptor-2
-
- ICC
-
- intrahepatic cholangiocarcinoma
-
- IGF
-
- phosphatidylinositol 3-kinase
-
- MAPK
-
- mitogen-activated protein kinase
-
- mTOR
-
- mammalian target of rapamycin
-
- PD-1
-
- programmed cell death protein 1
-
- PD-L1
-
- programmed cell death protein ligand-1
-
- PI3K
-
- phosphatidylinositol 3-kinase
-
- PIK3CA
-
- phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha
-
- SH
-
- steatohepatitic
-
- SOAT1
-
- sterol O-acyltransferase 1
-
- TCF-1
-
- HNF1 homeobox A
-
- TCGA
-
- The Cancer Genome Atlas
-
- TERT
-
- telomerase reverse transcriptase
-
- TGF-β
-
- transforming growth factor-beta
-
- TP53
-
- tumor protein p53
-
- TSC1/2
-
- tuberous sclerosis complex 1 and 2
-
- USFDA
-
- U.S. Food and Drug Administration
1 INTRODUCTION
Hepatocellular carcinoma (HCC) is the most frequent type of primary liver cancer (approximately 90%). Globally, it is the eighth most prevalent and the third leading cause of cancer [1, 2]. China has the highest morbidity and mortality burden of HCC, accounting for over half of both new cases and deaths associated with the disease [3-5]. In China, HCC ranks first both in incidence and mortality in males under 60 years old [6]. Several risk factors, such as cirrhosis, hepatitis virus infection, alcohol abuse, metabolic syndrome, and aflatoxin B1 exposure, have been identified for HCC [7-9]. Evidence from epidemiological studies demonstrates that non-alcoholic fatty liver disease [10, 11], diabetes [12, 13], and tobacco usage [14, 15] increase the risk of HCC. In China, among all cancers, HCC has the lowest survival rate, with the 5-year overall survival rate below 10% [6]. This has been ascribed to several challenges, among which the late diagnosis resulting in the disease mainly detected at advanced stages. In addition to this, high malignancy is another factor attributed to the low overall survival rate of HCC patients, shown to limit the efficacy of therapeutics [16].
The current systems used for HCC subtyping are largely based on tumor burden, which provide a moderate classification of tumors [17]. Meanwhile, the Barcelona Clinic Liver Cancer (BCLC) system is so far the most commonly used model for predicting the prognosis and guiding the selection of therapeutic interventions for HCC. According to this system, treatment options, such as ablation, resection, and transplantation, among others, are effective interventions for early HCC [18], however, they are less effective for advanced stage HCC [19]. Notably, the BCLC staging system is not sufficiently sensitive and specific to delineate the biological and molecular characteristics that influence prognosis and response to therapy, particular among tumors of the same stage [20]. Intriguingly, Nault et al. [21] showed that tumors at the same stage may display diverse genetic blueprints. Thus, it is imperative to develop more precise subtyping methods. Here, we summarize the current research progresses in precision therapy on the backbone of molecular subtypes of HCC.
2 GENOMIC HETEROGENEITY AND PRECISION THERAPY
HCC is a highly heterogeneous disease both at the pathological and molecular levels, stratified into intra- and inter-tumor heterogeneity [22-24]. These heterogeneities may be related to etiological diversity, morphological diversity, genetic predisposition, mutational alterations, dysregulation of signaling pathways, and micro-environmental disturbances [25-28]. Although the advances in next-generation sequencing technology have revealed new insights into the genome-wide landscape of HCC [29-32], molecular features and pathogenesis of HCC are not fully known. Precision medicine approach provides more accurate classification and diagnosis of cancer at the molecular level based on the patient's internal biological information and clinical characteristics [33-35]. On the backbone of large genetic information and clinical data, precision medicine is a key component of effective treatment [36, 37]. Given the highly heterogeneous genomic aberrations and microenvironment in HCC, it is challenging to explicitly simulate how target treatments kill a single cell. Hence, grouping cancer patients into relative homogeneous subtypes based on critical features, such as human epidermal growth factor receptor-2 (HER2)-positive gastric cancer [38] and estrogen receptor-positive breast cancer [39], may offer a great clinical significance. In the following sections, we describe the current progress in molecular subtyping of HCC and how it has revolutionized precision medicine.
3 MOLECULAR ALTERATIONS IN HCC
HCC results from the accumulation of multiple genomic and epigenomic alterations. Even though approximately 40-60 somatic alterations have been detected in exons of the HCC genome [9], only a few of them alter key signaling pathways that drive hepatocarcinogenesis. In fact, most frequently altered genes in HCC, such as telomerase reverse transcriptase (TERT), tumor protein p53 (TP53), and catenin beta-1 (CTNNB1), remain as unactionable targets [32]. Epigenetic dysregulation also plays a crucial role in hepatocarcinogenesis by altering gene expression through various mechanisms, including induction of DNA methylation, modification of histones, chromatin remodeling, and altering the expression of non-coding RNAs (microRNAs and long intergenic non-coding RNAs) [23]. Next-generation sequencing technologies have revealed several signaling pathways and oncogenic alterations associated with HCC, such as telomere maintenance, cell cycle control, epigenetic modifiers, WNT/β-catenin pathway, mammalian target of rapamycin (mTOR) and mitogen-activated protein kinase (MAPK) kinase pathway [7, 40].
4 TARGETED THERAPY BASED ON MOLECULAR FEATURES OF HCC
Sorafenib was the first drug for targeted therapy approved by the U.S. Food and Drug Administration (USFDA) for advanced HCC. It delays hepatocarcinogenesis by blocking tumoral angiogenesis and cell proliferation [41]. Hitherto, four molecular targeted agents, sorafenib [42, 43] and lenvatinib [44] for first-line together with regorafenib [45] and cabozantinib [46] for second-line treatment, have displayed promising benefits in phase III clinical trials for advanced HCC. However, they are associated with adverse reactions, suboptimal efficacy, and frequent resistance [47].
Future trials are therefore needed to test various combinations of multi-kinase inhibitors to provide alternative drugs for patients who fail or do not tolerate first- and second-line therapies. By June 2016, the USFDA had approved 72 targeted anti-tumor drugs, including rapamycin and everolimus (mTOR inhibitors), perifosine and deguelin (AKT kinase inhibitors), tipifarnib (a RAS signaling inhibitor), and wortmannin (a phosphatidylinositol 3-kinase (PI3K) inhibitor). As an example, an HCC patient presented with distant metastases 11 years after liver transplantation. Immunohistochemistry tests performed on tumor cells revealed that this patient was positive for nuclear β-catenin (the WNT pathway) and pS6 (the mTOR pathway). The patient failed to respond to sorafenib alone and was therefore put on everolimus (an mTOR inhibitor). Three months later, the tumor had diminished by nearly half [48]. This case reveals that targeting relevant molecular pathways is effective in treating recurrent HCC. However, there was no evidence to suggest that a combination therapy of everolimus and sorafenib was superior to sorafenib therapy alone in patients with advanced HCC [49]. Overall, the current targeted drugs have only been shown to be effective only in a small portion of patients, hence are not ideal for precision medicine [50]. Studies conducted in the past decade have shed light on the molecular pathogenesis of HCC. Despite this, current therapeutic options are not sufficiently effective, and no biomarker for any approved targeted drug has been defined [51].
Immunotherapy, which remotivates intrinsic immune response to attack tumors by targeting relevant regulatory pathways against immunocytes, has shown remarkable efficacy on different solid tumors [52-54]. Immune checkpoint inhibitors, such as anti-programmed cell death protein 1 (PD-1) and anti-cytotoxic T lymphocyte-associated protein 4 (CTLA-4) antibody have been approved for the treatment of several tumors [55]. For instance, phase II studies on nivolumab and pembrolizumab (anti-PD-1) for second-line treatment of advanced HCC revealed that the objective response rate and prognosis are promising [56, 57]. However, the heterogeneous immune profile of HCC coupled with high immunotolerant environment limit the efficacy of immunotherapies [58, 59]. Consequently, further researches are required to identify patients likely to benefit from immunotherapy.
5 SUBTYPING OF HCC BASED ON MOLECULAR FEATURES
Several multi-omics technologies have been used to characterize the mutational, transcriptomic, proteomic, metabolic, and immunological alterations in HCC. This has promoted the application of Big Data in the molecular subtyping of HCC [60]. Here, certain molecular signatures reflect specific tumor phenotypes, such as microvascular invasiveness and aggressiveness [61]. This underlines the invaluable benefits of molecular characterization and classification of tumors in the application of precision medicine. To meet these clinical objectives, multiple schemes of HCC sub-grouping have been proposed. The framework of these systems is based on different levels last decades. In the first foundation stage, the aim is establishing single-omic unsupervised molecular subtypes based on apparent biological characteristics and molecular features. The second phase involves molecular subtyping based on multi-omics spectral profiling. Together, precise subtyping with clinical significance is achieved. The following subsections summarize the major recent advances in molecular subtyping of HCC and probable future applications of the molecular feature-based precision medicine (Figure 1).
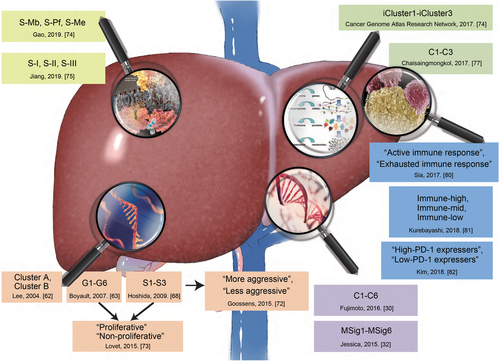
Advances in molecular subtyping of HCC. Current researches on the molecular subtyping of HCC have been focused on single-omic (transcriptomic, mutational, and proteomic subtyping), multi-omic, and immune subtyping. These approaches have created a window for developing therapy based on molecular subtyping.
Abbreviations: HCC, hepatocellular carcinoma; PD-1, programmed cell death protein 1
5.1 Transcriptomic HCC subtyping
In this subsection, we review studies that employed global transcriptome and meta-analyses to investigate the correlation between transcriptome and genotype, as well as those that delineate different HCCs based on specific genetic identifiers.
First, Lee et al. [62] proposed two distinctive HCC subgroups (Clusters A and B) based on gene expression patterns in 91 HCCs. Here, tumors in Cluster A displayed cell proliferation and antiapoptosis gene expression signatures, while tumors in Cluster B displayed up-regulated expression of genes involved in ubiquitination and histone modification. Meanwhile, Boyault et al. [63] identified 6 HCC subgroups (G1-G6). G1 tumors were associated with low copy number of hepatitis B virus (HBV), overexpression of fetal liver genes, and activated AKT pathway. G2 tumors displayed high copy number of HBV, mutations in phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha (PIK3CA) and TP53 genes, as well as activated AKT pathway. G3 tumors were characterized by TP53 mutations, but were conspicuously not observed in patients with HBV infection. In addition, there was overexpression of cell cycle-related genes. G4 tumors were a heterogeneous subgroup comprising of adenomas and carcinomas characterized by HNF1 homeobox A (TCF-1) mutations. G5 tumors were closely associated with activated β-catenin/WNT pathway, whereas, in addition to the characteristics of G5 tumors, G6 tumors were characterized by the expression of more satellite nodules and higher activation of β-catenin. Furthermore, G3's specific molecular feature was an independent predictive risk factor for early recurrence [64]. Building on these findings, Calderaro et al. [65] proposed the Boyault's typing by integrating histological and clinical features to discern the molecular and histopathological correlations. Additionally, another research in Korea validated the HCC G1-G6 typing in individuals of Asian and European ancestries [66]. Elsewhere, Chiang et al. [67] combined copy number alterations and gene expression profiles in 91 tumors and identified 5 classes, including ‘‘CTNNB1’’, ‘‘proliferation’’, ‘‘interferon-related’’, “polysomy of chromosome 7”, and an unannotated class. Characteristics of tumors in CTNNB1 and proliferation subgroups displayed multiple similarities with Lee's [62] and Boyault's typing [63].
After performing a meta-analysis of gene expression profiles involving 603 HCC samples, Hoshida et al. [68] identified 3 robust subclasses (S1-S3), each associated with distinct clinical histopathological features. S1 tumors were characterized with aberrant activation of transforming growth factor-beta (TGF-β) and WNT pathways, whereas S2 tumors exhibited activated MYC and AKT pathways and overexpression of stemness markers, such as α-fetoprotein (AFP) and epithelial cell adhesion molecule (EPCAM). Meanwhile, S3 tumors were smaller, well-differentiated, and overexpressing genes related to liver function (e.g., glycolipid metabolism related genes). With regard to prognosis, up-regulated AFP was associated with aggressive tumor and poor clinical outcome [69]. The S2 subtype was also associated with poor prognosis. For HCC patients not fitting in the Milan sub-grouping criteria and not displaying S2 signatures but underwent liver transplantation, their survival rates was comparable to those fitting in Milan sub-grouping [70]. Building on previous works, Tan et al. [71] established clinicopathological predictive indices which served as surrogate markers for S1-S3 typing, including steatohepatitic (SH)-HCC variant and immune cell infiltration for the S1 subtype, macrotrabecular pattern, lack of pseudoglandular pattern, and high serum AFP (>400 ng/mL) for the S2 subtype, and microtrabecular pattern, lack of SH-HCC, and low histological grade for the S3 subtype.
Based on the S1-S3 typing, Goossens et al. [72] further grouped HCC into “More aggressive” and “Less aggressive” subtypes based on signaling pathways, biological characteristics, and clinical outcome. Here, S1 and S2 tumors were characterized by TP53 mutations and activation of pro-oncogenic signaling pathways (e.g., TGF-β and MET), expression of stemness markers (e.g., EPCAM), and poor differentiation and were associated with high recurrence and low survival rates, whereas S3 tumors were featured by CTNNB1 mutations, overexpression of liver-specific WNT targets, and better differentiation and were associated with low recurrence and high survival rates.
Based on previous HCC molecular subtyping systems of Boyault et al. [63] and Hoshida et al. [68], Llovet et al. [73] proposed a secondary classification of tumors designated as “Proliferative” and Non-proliferative”. The proliferative subclass was associated with HBV-related etiology, characterized by high activation of classic RAS, mTOR, and Insulin like growth factor (IGF) signaling, and associated with poor outcome. They further stratified the subclass in two phenotypic tertiary subtypes: the WNT/TGF-β subtype, characterized by activated TGF-β and WNT pathways, and the progenitor subtype, which overexpressed hepatic progenitor markers. The non-proliferative subclass is a group of heterogeneous cancers associated with alcohol consumption and hepatitis C virus (HCV) infection. Meanwhile, some patients share CTNNB1 mutations and have common molecular profile.
5.2 Mutational HCC subtyping
To understand the genomic alterations in tumors, it is critical to identify driver mutations which can be potentially targeted to improve patient's survival. High-throughput sequencing technologies, such as whole-genome and exome sequencing, have led to the identification of two subtyping methods.
Schulze et al. [32] identified 161 putative driver genes and 11 altered pathways based on exome sequencing analysis of 243 HCC samples, including TERT activation of telomerase, TP53 inactivation, and alterations of WNT, mTOR, MAPK, and TGF-β pathways. Using mutational signatures, they identified 6 groups of tumors (MSig1-6). The specific characteristics of each group are as follows: MSig1 with frequent C>A and dinucleotide mutations; MSig2 with a high rate of C>A mutations, HBV infection, and induced by Aflatoxin B1; MSig3 with alcohol and tobacco exposure, CTNNB1 mutation, and non-cirrhotic liver; MSig4 with frequent TP53 mutations, enriched in T>C transitions; MSig5 with low mutation rate and early histological stage; MSig6 with TERT and CTNNB1 mutations.
Fujimoto et al. [30] provided a whole-genome landscape of 300 HCCs from which 6 clusters (C1-6) of HCC were identified based on the mutations of specific driver genes: C1 —— ARID2, PBRM1 (PBAF complex); C2 —— LRP1B, ARID1A, PTPRD, RB1, APOB; C3 —— MACROD2; C4 —— CTNNB1; C5 —— CDKN2A; C6 —— TP53. C1 and C6 appeared to have worse prognosis, whereas C3 showed better prognosis.
5.3 Proteomic HCC subtyping
Proteomics is the study of the characteristics of proteins at a large-scale level, ranging from protein expression and post-translational modifications. It also aims to understand the pathogenesis of diseases and cell metabolism at the protein level. The direct targets of multi-kinase inhibitors are proteins. Recent studies have expanded our understanding of the proteomic profile of HCC which is likely to benefit disease treatment.
Gao et al. [74] performed proteogenomic characterization on 159 patients with HBV-related HCC. They identified 3 subgroups with distinct signaling features and metabolic pathways. The metabolic subgroup had the highest level of metabolism-related proteins and liver function retention. The proliferation subgroup displayed up-regulated expression of proliferation-associated proteins and shortest overall survival. The microenvironmental dysregulation subgroup exhibited down-regulated expression of immunity, inflammatory, and stromal proteins.
Jiang et al. [75] used quantitative proteomic data of 110 early-stage tumors to stratify the cohort into the subtypes S-I, S-II, and S-III. In S-I tumors, liver function-related proteins and pathways were exclusively up-regulated, suggesting that these tumors have hepatocyte-like characteristics. S-II and S-III tumors were both characterized by elevated levels of proliferation-related proteins and pathways. S-III tumors exhibited a higher level of immune infiltration and more aggressive characteristics compared with S-I and S-II, which were associated with a poor prognosis. Interestingly, S-III tumors specifically expressed high level of sterol O-acyltransferase 1 (SOAT1), the knockdown of which altered the distribution of cellular cholesterol and effectively suppressed the progression of cancer.
5.4 HCC subtyping based on multi-omics
Integrated monitoring analysis of genetic mutations, transcriptome, epigenetics, proteomics, and metabonomics may reveal the intrinsic molecular characteristics of tumors. Analysis of multi-omics datasets has helped to identify the presence of different molecular subtypes of tumors. The Cancer Genome Atlas (TCGA) research network which allows unsupervised clustering from 5 platforms, including DNA copy number, methylation, mRNA, microRNA, and protein array, revealed 3 major subtypes (iCluster1-3) of HCC [76]. ICluster1 exhibited a high tumor grade and the presence of macrovascular invasion with significantly worse prognosis. Its molecular features included low frequencies of CTNNB1 and TERT promoter mutations and overexpression of proliferation marker genes, corresponding to Hoshida's S2 subtype. It also exhibited specific changes in lipid metabolism-related microRNA expression. ICluster2 and iCluster3 exhibited high frequencies of DNA hypermethylation-caused CDKN2A silencing, TERT promoter mutations, and CTNNB1 mutations. ICluster2 was clinically characterized by low-grade tumors and less microvascular invasion, corresponding to Hoshida's S3 subtype. ICluster3 was characterized by a high degree of chromosomal instability, high frequency of TP53 mutation, and hypomethylation of multiple CpG sites, corresponding to the Hoshida's S1 subtype.
Integrated multi-omics analysis has also uncovered similarities and differences among HCC samples, intrahepatic cholangiocarcinoma (ICC), and combined/mixed HCC-ICC, which are usually considered as different entities. Chaisaingmongkol et al. [77] integrated genome, transcriptome, and metabolome profiles of 199 Asian HCC samples, including both HCC and ICC. They identified three HCC subtypes (HCC C1-3) and four ICC subtypes (ICC C1-4). Interestingly, they demonstrated significant similarity in expression and prognosis between HCC-C1 and ICC-C1 (common C1) and between HCC-C2 and ICC-C2 (common C2). Common C1 subtype was highly enriched with mitotic checkpoint signaling pathways and Hoshida's S1-2 subtype-related genes, whereas common C2 subtype was highly enriched with cell immunity-related pathways. A recent study showed that mixed HCC-ICC shared more common molecular features with HCC, while combined HCC-ICC showed similar features to ICC [78].
5.5 Immune HCC subtyping
Given the inconsistent efficacy of immune therapy, it is imperative to select patients who are likely to clinically benefit from immune checkpoint inhibitors based on immune subtypes and immune microenvironment profiles. PD-1 is a key immune checkpoint receptor expressed on activated T cells which mediates immunosuppression [79]. Immunotherapy works by harnessing antitumor immune response [55].
In 2017, Sia et al. [80] characterized the molecular features of infiltrated immune cells. Specifically, they analyzed the gene expression data of 956 HCC samples and its association with immunologic landscape. Their results showed that approximately 25% of HCCs showed high expression of inflammatory response markers, such as PD-1 and programmed cell death protein ligand-1 (PD-L1). Moreover, they identified 2 subclasses characterized by active and exhausted immune responses. The exhausted immune response subclass expressed many genes regulated by TGF-β1 that regulate immunosuppression. The active immune response subclass displayed significant enrichment of T cells and IFN signatures, and showed similar high overall survival rate. Subsequently, Kurebayashi et al. [81] explored the heterogeneity of 158 HCC samples and classified immune microenvironment into 3 subtypes: immune-high, immune-mid, and immune-low. The immune-high subtype was characterized by increased B/T-cell infiltration, poor differentiation, increased PD-1/PD-L1 positivity in CD8+ T cells and tumor cells, and was significantly associated with Hoshida's S1 and Boyault's G2 subtypes. The immune-mid subtype was characterized by moderately increased T-cell and other immune cell infiltration with lesser B-cell infiltration. Differences in PD-1 expression patterns have been reported in exhausted tumor-infiltrating CD8+ T cells. In the PD-1-high subpopulation, Kim et al. [82] identified 2 subgroups termed “High-PD-1 expressers” and “Low-PD-1 expressers”. “High-PD-1 expressers” were associated with more aggressive biological tumor characteristics, expressing multiple immune checkpoint receptors which predicted a favorable response to anti-PD-1 therapy. Overall, HCC patients with high proportions of PD-1-high CD8+ T cells might be more susceptible to immune checkpoint inhibitors.
Together, the 3 subtypes exhibit a panorama of HCC immune microenvironment, suggesting that immune subtyping has potential clinical value for patient stratification and development of novel subtype-based therapies.
6 ADVANTAGES AND LIMITATIONS OF SUBTYPING METHODS
The molecular subtyping of HCC based on the aforementioned studies highlights the value of large-scale “-omics” in classification of tumors. Therefore, omics may help the design of potential treatment opportunities that are customized to a homogeneous subgroup of cancer. Molecular subtyping may likely become a common tool for HCC diagnosis and characterization during clinical decision making. However, differences in technological platforms, patient population, preparation and processing of samples make it difficult to develop a common method for classification of HCC tumors. Notably, some subtypes have been repeatedly discovered in independent studies, meaning that different HCC subtypes derived from different subtyping methods may share common molecular features (Figure 2). Given that similarities among independent subtypes still remains superficial, more studies should be conducted to create consensus among different subtyping methods.
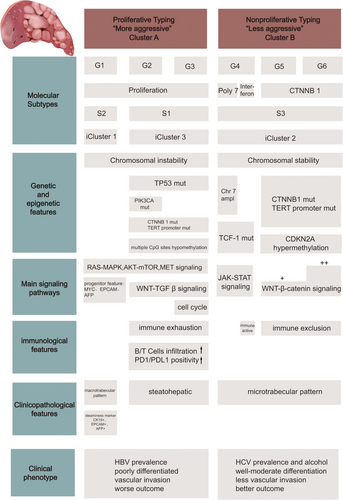
The concordance between different molecular subtyping methods for HCC. The main molecular subtypes of HCC shown are derived from “-omic”-based subtyping methods previously reported in 7 studies (Lee et al. [62], Boyault et al. [63], Chiang et al. [64], Hoshida et al. [68], Goossens et al. [72], Lovet et al. [73], and Cancer Genome Atlas Research Network [76]). For each subclass, major associations with genetic, epigenetic, pathways, etiologies, immunological and clinical phenotypes are shown.
Abbreviations: HCC, hepatocellular carcinoma; CTNNB1, catenin beta-1; TP53, tumor protein p53; TERT, telomerase reverse transcriptase; TCF-1, HNF1 homeobox A; EPCAM, epithelial cell adhesion molecule; AFP, α-fetoprotein; PD-1, programmed cell death protein 1; PD-L1, programmed cell death protein ligand-1; HBV, hepatitis B virus; HCV, hepatitis C virus
7 MOLECULAR SUBTYPE-GUIDED PRECISE TREATMENT
Currently, little is known about the molecular subtypes of HCC to allow effective clinical management of HCC. Integrative experimental and genomic analyses have revealed clinically relevant HCC subtypes and characteristic genomic alterations associated with the subtypes. It has been found that different subtypes of HCC cell lines showed distinct responses to molecular targeted agents. By integrating gene expression pattern and analyzing cellular origins of tumors, Lee et al. [83] identified a subtype of HCC arising from hepatic progenitor cells called “Hepatoblast subtype”, another subtype arising from other hepatic cells named “Hepatocyte subtype”. A Src/Abl small-molecule kinase inhibitor, dasatinib, exhibited preferential killing of the hepatoblast subtype cell lines [84]. The Hoshida's S2 subtype may also be an important biomarker reflecting response to target therapy. Activation of MYC pathway S2 subtype cell lines increased the sensitivity to a small-molecule BET bromodomain inhibitor, (+)-JQ1, which suppressed the activity of MYC. Inhibitors of the fibroblast growth factor receptors (FGFRs) blunted MAPK signaling in S2 cell lines [85].
The results from analysis of genomic data have started to impact clinical decision making and the development of more precise therapies. Many clinical studies have attempted to stratify patients based on biomarkers for precision treatment. In a retrospective analysis of phase II study, patients with advanced HCC harboring RAS mutations exhibited the best clinical responses to a combination of refametinib (MEK inhibitor) and sorafenib [86]. Subsequent studies obtained similar results, but further studies are needed since those studies were performed on few cases [87]. Recently, Sun et al. [88] identified Fms-like tyrosine kinase 3 (FLT3) as a predictive biomarker of sorafenib in advanced HCC. It was found that sorafenib may delay tumor progression in patients with FLT3-high HCC. Tuberous sclerosis complex 1 and 2 (TSC1/2) are mTOR pathway-related genes that are frequently mutated. Mutations of TSC1/2 decreased the activity of mTOR pathway and promoted malignant progression of HCC [89]. Ho et al. [90] identified a novel molecular subgroup of HCCs with aggressive behaviors, harboring TSC1/2 mutations and showing hypersensitivity to rapamycin treatment. However, many targeted drugs lack biomarkers to demonstrate association of genomic subtypes with treatment response.
Although most of these findings are encouraging, there are substantial gaps in translating molecular subtypes to clinical practice [91]. Patient-derived xenograft model is one of the most preferred preclinical models that best recapitulates hepatocarcinogenesis and histologic aggressiveness of different subtypes of HCC [92]. Preclinical models provide a framework that guides preclinical trials of novel drugs, thereby bridging the link between molecular subtypes and precision therapy.
8 PERSPECTIVE
Identification of HCC subtypes have generated new ideas for patient stratification and revealed opportunities for developing therapeutic agents for personalized curative-intent treatment (Figure 3). Molecular subtyping-based analyses of HCC samples have expanded our understanding of the pathogenesis of HCC and provided a basis for clinical subtyping of tumors which have improved treatment of HCC patients. This review reveals that the design of targeted therapies may be visible if sufficient information on the clinicopathological features [93] and the concept of molecular subtyping are optimally utilized in clinical settings. In the foreseeable future, more researches and collaborations are required to apply multi-omics tools to identify the mechanisms of HCC and to evaluate efficacy of novel drugs targeted at molecular pathways in specific subgroups of HCCs patients [94]. Relatively aggressive subtypes of tumors can be converted into another less aggressive subtype through subtype-targeted strategies, e.g., targeting oncogenic pathway. Further advanced molecular monitoring and examination of tumor microenvironment may identify more accurate predictive and prognostic biomarkers of HCC. To produce clinically translatable findings, animal models which reproduce human HCC's specific molecular traits and tumor phenotypes are needed. Finally, prospective clinical trials organized by multi-centers should be conducted to assess subtype-specific responses to therapy.
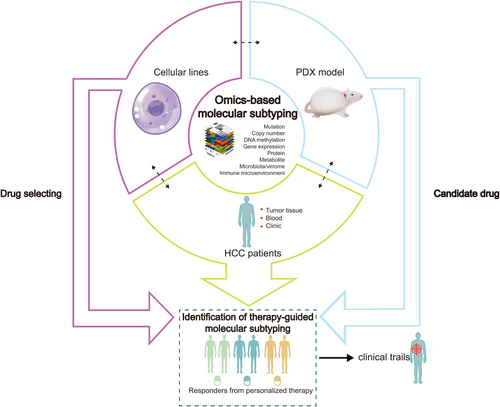
An algorithm for precise treatment of HCC. The development of new -omic technologies, multi-dimensional and multi-level comprehensive analyses of HCC have enabled classification of HCCs into distinct subgroups, thereby improving clinical management of HCC.
Abbreviations: HCC, hepatocellular carcinoma; PDX, patient-derived xenograft.
DECLARATIONS
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
COMPETING INTERESTS
The authors declare that they have no competing interests.
FUNDING
This work was supported in part by the grant from the National Science and Technology Major Project of China (No. 2017ZX10203205), the Science Foundation of Zhejiang province (LQ18H160006).
AUTHORS' CONTRIBUTIONS
Y.W. collected the related references and drafted the manuscript. Z.L. revised the manuscript and drafted the figures. X.X. designed the review and drafted the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
Not applicable.




