Favorable response to immunotherapy in a pancreatic neuroendocrine tumor with temozolomide-induced high tumor mutational burden
Abstract
Neuroendocrine neoplasm of the pancreas is a rare tumor with limited treatment options. Among such tumors, treatment for pancreatic neuroendocrine tumor (PanNET) G3 is the most difficult. Temozolomide (TMZ) is commonly used to treat PanNET. However, TMZ may cause tumor gene alkylation, which induces drug resistance and rapid disease progression. Herein, we present a case of a female who was diagnosed with PanNET G3 and achieved a partial response to toripalimab, an anti-programmed cell death-ligand 1 (anti-PD-L1) monoclonal antibody, after multiple cycles of TMZ treatment. Genomic profiling revealed that compared with the patient's samples collected at baseline, the post-TMZ-treatment samples had markedly higher levels of tumor mutational burden (TMB) associated with characteristic alkylating mutational signature representing a positive correlation with favorable response to anti-PD-1 treatment. In addition, we observed a germline truncating mutation of MUTYH (W156*) that was considered to be pathogenic and potentially conferred to genomic instability. This case suggests that anti-PD-1 therapy could be a treatment option for PanNET patients with increased TMB after TMZ-based treatment.
Abbreviations
-
- AF
-
- allele frequency
-
- ATRX
-
- ATRX chromatin remodeler
-
- CAPTEM
-
- capecitabine/temozolomide
-
- ctDNA
-
- circulating tumor DNA
-
- FACETS
-
- fraction and allele-specific copy number estimates from tumor sequencing
-
- ICIs
-
- immune checkpoint inhibitors
-
- IHC
-
- immunohistochemical
-
- LOH
-
- loss of heterozygosity
-
- NGS
-
- next-generation sequencing
-
- PanNECs
-
- pancreatic neuroendocrine carcinomas
-
- PanNENs
-
- pancreatic neuroendocrine neoplasms
-
- PanNETs
-
- pancreatic neuroendocrine tumors
-
- PD
-
- progressive disease
-
- PD-1
-
- programmed death receptor-1
-
- PET/CT
-
- positron emission tomography/computed tomography
-
- PR
-
- partial response
-
- RB1
-
- RB transcriptional corepressor 1
-
- SSA
-
- somatostatin analogs
-
- SSTR
-
- somatostatin receptor
-
- TAE
-
- tumor arterial embolization
-
- TMB
-
- tumor mutational burdenBMI, body mass index
1 INTRODUCTION
Pancreatic neuroendocrine neoplasms (PanNENs) are morphologically grouped into well-differentiated neuroendocrine tumors (PanNETs) and poorly-differentiated neuroendocrine carcinomas (PanNECs) [1]. NETs are graded as G1, G2, or G3 based on their proliferative activity [1]. Besides immunohistochemistry (IHC) tests, genetic tests also play important roles in PanNENs diagnosis [2].
Treatments for unresectable or metastatic PanNENs depend on the tumor pathology [3], while the treatment for well-differentiated PanNET G3 follows that of PanNET G2, i.e. use of the capecitabine/temozolomide (CAPTEM) regimen [4], and second-line treatments including everolimus [5] and sunitinib [6]. For poorly-differentiated PanNET G3, platinum-based chemotherapy is often preferred [7]. Currently, immunotherapy is not yet approved for PanNETs as clinical trial data have suggested an unpromising response [8], but recently a 75% objective response rate was reported in NET with high tumor mutational burden (TMB) (≥ 9.9 mutations/Mb) [9].
Here, we present the case of a patient diagnosed with metastatic PanNET G3 who had characteristic gene mutations along multiple lines of treatments.
2 CASE REPORT
A 49-year-old female (Figure 1A) (BMI, body mass index; 16.82) presenting epigastric pain underwent magnetic resonance imaging, which showed a tumor at the pancreatic tail, with enlarged regional lymph nodes, and tumor thrombosis within the portal and splenic vein (Figure 1B). An ultrasound-guided biopsy of the pancreatic tumor revealed a well-differentiated NET (Figure 2A) with high proliferation (Ki-67 index: 35%+; Supplementary Table S1). 68Ga-DOTATATE-PET/CT showed a high expression of the somatostatin receptor (SSTR) in all lesions. After a multi-disciplinary team discussion, the patient was diagnosed with PanNET G3 on January 22, 2018. Comorbidity included hyperlipidemia and was treated with atorvastatin.
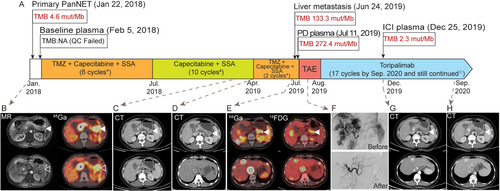
Chronological presentation of the patient's clinical history and medical treatment. (A) Medical history of the patient. (B) MR: fullness of the pancreatic body and tail, with a mass of long T2 signal and uneven enhancement, 25 mm × 23 mm in size (solid arrowhead: primary pancreatic tumor), without a clear demarcation line between the tumor, the spleen, and the wall of the gastric body. The hollow arrowheads indicate multiple enlarged adenopathies adjacent to the pancreatic mass. The asterisk points to the tumor thrombosis characterized by the dilation of the portal vein and splenic vein, with massive long T1 and uneven long T2 signal inside (maximum diameter of 33 mm). 68Ga: A mass at the pancreatic body and tail, with multiple adenopathies; tumor thrombosis within the portal vein and splenic vein. All lesions showed a high expression of SSTR (SUVmax, 37.6). (C) CT: A locally invasive tumor mass at the level of the pancreatic body and tail (solid arrowhead: primary pancreatic tumor), 23 mm × 20 mm in size. (D) CT: A locally invasive tumor mass at the level of the pancreatic body and tail (solid arrowhead: pancreatic primary), 24 mm × 20 mm in size. Multiple intrahepatic foci of varying sizes and low density (hollow circles: liver metastases), with the largest one 28 mm × 24 mm in size, and the smallest one being 5 mm in size. (E) 68Ga and 18FDG: A mass at the level of the pancreatic body and tail, with multiple intrahepatic foci of varying sizes. All lesions show a high expression of SSTR (SUVmax 33.1) and moderate 18FDG uptake (SUVmax 7.2). (F) Hepatic arteriograms before and after transarterial embolization show that the feeding artery of the tumor was completely blocked. (G) CT: A locally invasive tumor mass at the level of the pancreatic body and tail (solid arrowhead: primary pancreatic tumor), 33 mm × 17 mm in size. Filling defect at the level of the main portal vein and splenic vein (asterisk: tumor thrombosis). Multiple liver nodules show iodine deposition which was caused by TAE (hollow circle: liver metastasis), the largest one 12 mm × 10mm in size. (H) CT: A locally invasive tumor mass at the level of the pancreatic body and tail (solid arrowhead: primary pancreatic tumor), 33 mm × 17 mm in size. Filling defect at the level of the main portal vein and splenic vein (asterisk: tumor thrombosis). Multiple liver nodules show iodine deposition (hollow circle: liver metastasis), the largest of which was 10 mm × 9 mm in size. Detailed dosages of medications: *Capecitabine 1 g/m2 (1 g Qd 1.5 g Qn), d1-14; TMZ 0.26 g Qd d10-14; SSA 30 mg im d1 Q28d; # Capecitabine 1 g/m2 (1 g Qd 1.5 g Qn), d1-14; SSA 30 mg im d1 Q28d; П Toripalimab 240 mg iv d1 Q21d. Accumulated TMZ dose 7.54 g/m2. Abbreviations: PanNET: pancreatic neuroendocrine tumor; TMB: tumor mutational burden; NA: not applicable; QC: quality control; TMZ: temozolomide; SSA: somatostatin analogs; PD: progressive disease; ICI: immune checkpoint inhibitor; MR: magnetic resonance; 68Ga: Ga-68 DOTATATE positron emission tomography/computed tomography; SSTR: somatostatin receptor; CT: computed tomography; 18FDG: F-18 fluorodeoxyglucose positron emission tomography/computed tomography; TAE: tumor arterial embolization
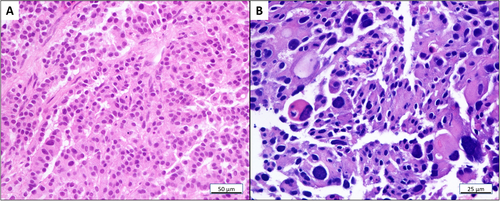
The hematoxylin and eosin stain of (A) PanNET primary and (B) liver metastasis. (A) PanNET primary tumor at baseline (magnification: × 200) exhibits an organoid and trabecular architecture, a regular intratumoral vascular pattern, abundant granular cytoplasm, and stippled nuclei with inconspicuous nucleoli; (B) liver metastasis (magnification: × 400) exhibits a composition of large nests of cells with prominent atypia. Abbreviations: PanNET: pancreatic neuroendocrine tumor
First-line treatment with six cycles of CAPTEM plus somatostatin analogs (SSA) (capecitabine 1 g Qd 1.5 g Qn, d1-14; TMZ 0.26 g Qd d10-14; SSA 30 mg d1 Q28d) led to a partial response (PR) and relief of symptoms. Adverse events included grade 2 leukopenia and thrombocytopenia. Following that, 8 cycles of capecitabine plus SSA were administered as maintenance therapy and follow-up CT scans revealed stable disease (Figure 1C). However, after the tenth cycle of maintenance treatment, multiple new liver lesions were discovered ranging from 5 mm to 28 mm (Figure 1D). Given the favorable response of the first-line treatment, two cycles of CAPTEM plus SSA were re-challenged, but both 68Ga-DOTATATE and 18FDG-PET/CT showed progressive disease (PD) (Figure 1E). An ultrasound-guided biopsy of the liver lesions was performed and showed higher proliferation (Ki-67 index: 80%+; Supplementary Table S1) compared to the primary PanNET (Figure 2B). Since the pancreatic lesion remained stable, re-biopsy was avoided to reduce unnecessary injuries.
Tissue samples of the primary PanNET, liver metastasis and plasma samples collected at baseline and after PD (Figure 1A) were subjected to targeted next-generation sequencing (NGS; Nanjing Geneseeq Technology Inc., Nanjing, Jiangsu, China) for 425 cancer-relevant genes, as described previously [10]. Circulating tumor DNA (ctDNA) mutation positivity increased upon PD (Figure 3A), and the TMB in PD plasma and liver metastasis samples increased dramatically (Figure 1A), and was found to be associated with the alkylation-induced mutational signature, Signature 11 (Figure 3B), similar to a previous report [11]. However, the mutation signature could not be identified in baseline samples due to the low mutation number (< 5 mutations). Moreover, a germline heterozygous nonsense mutation of MUTYH (p.W156*) was identified by NGS in all plasma collected samples (Supplementary Table S2). Fraction and allele-specific copy number estimates from tumor sequencing [12] analysis showed that loss of heterozygosity (LOH) was common in the primary PanNET, including chromosome 1p, where the MUTYH gene is located (Figure 3C). However, due to the low amount of ctDNA in the baseline plasma sample, we could not detect any mutations above the 0.5% cut-off adopted for the analysis, which was determined by the applied targeted NGS.
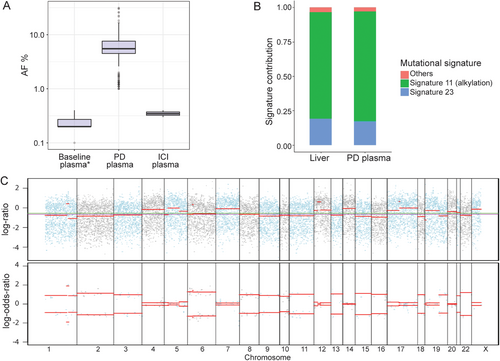
Disease surveillance and monitoring with NGS. (A) Mutational AF of ctDNA in plasma samples collected before TMZ treatment (baseline), after PD, and after ICI. The extracted amount of cell-free DNA from each plasma sample was 16.2 ng/mL at baseline, 13.5 ng/mL after PD, and 13.7 ng/mL after ICI. The AF in the baseline plasma was below the 0.5% cut-off for quality control which was determined as the limit of detection of plasma from healthy controls. (B) Mutational signature analysis of NGS data from liver metastasis and plasma samples after the failure of first-line TMZ treatment. Signature 11, an alkylation-induced signature, was the most dominant, followed by Signature 23 whose etiology remains unknown. (C) Integrated visualization of FACETS analysis of NGS data from the primary PanNET sample indicates genome instability with the losses of multiple chromosomes. The top panel displays the total copy number log-ratio which is computed from the total read count in the tumor versus normal tissues at all panel-covered genomic regions. The bottom panel displays the allele-specific log-odds-ratio of the variant allele read counts in the tumor versus normal samples. The blue and gray dots are the observed values, and the red bars are estimates. The chromosomes are separated by lines and alternated by blue and gray dots. Abbreviations: NGS: next-generation sequencing; AF: allele frequency; ctDNA, circulating tumor DNA; TMZ: temozolomide; PD: progressive disease; ICI: immune checkpoint inhibitor; FACETS: fraction and allele-specific copy number estimates from tumor sequencing; PanNET, pancreatic neuroendocrine tumor
As liver metastasis was the major tumor burden, tumor arterial embolization (TAE) was applied to the liver lesions (Figure 1F). The patient then received toripalimab (Shanghai Junshi Bioscience Co., Ltd in China), a programmed death receptor-1 (PD-1) monoclonal antibody, 240 mg every 3 weeks, and achieved a PR (Figure 1G) by the follow-up visit in December 2019. The TMB (Figure 1A) and mutational allele frequency (AF; Figure 3A) were dramatically reduced after immunotherapy (median AF of PD plasma vs ICI plasma: 5.49% vs 0.35%). The anti-PD-1 treatment is still ongoing and has been administrated for 17 cycles. As of the last follow-up on September 3, 2020 (BMI 17.58), no significant adverse effects were observed and CT scans revealed stable diseases at the primary PanNET and liver metastases (Figure 1H).
3 DISCUSSION
Pathological diagnosis of the primary PanNET in this case report was challenging as it contained features of both NETs and NECs. The evidence of PanNET includes pathological morphology, Ki-67 index, SSTR2 expression, and the presence of ATRX mutation. However, the IHC and NGS results revealed the loss of RB1 in the primary pancreatic tumor and liver metastasis, which is a feature of PanNECs [2]. To our knowledge, this is the first case reporting the co-existence of mutations in the ATRX and RB1 genes, which are routinely used to distinguish PanNETs and PanNECs However, the liver lesion was poorly differentiated with a high Ki-67 index that resembled NECs, suggesting a possible but rare transformation from NET G3 to NEC [1].
NGS analysis contributed to the pathogenic diagnosis of this case, as we identified an inactivating germline mutation in the base-excision-repair gene MUTYH which was reported as a novel pathogenesis in PanNETs [14]. However, whether this monoallelic germline MUTYH mutation is the trigger for the genome-wide LOH remains to be determined. To illustrate the biological mechanisms causing high TMB in PD samples, we performed mutational signature analysis based on the combinations of characteristic mutation types. Signature 11, which is associated with alkylation due to TMZ treatment, may have contributed the most to the elevated TMB in this case [15]. However, the high TMB might also be attributable to other unknown mechanisms as we only performed mutational signature analysis for the samples with TMB > 10 mutations/Mb to ensure reliability. Due to different DNA extraction techniques and insufficient ctDNA release, the discordance between tissue TMB and blood TMB was reported [13] and could explain the inconsistent TMB values detected in the liver tissue and PD plasma.
In conclusion, we described a PanNET G3 case, with increasing TMB induced by TMZ-related alkylation, which presented a favorable clinical response to anti-PD-1 treatment. Based on the described observations, we suggest that the use of ICIs should be further explored as a treatment option for PanNET patients with increased TMB following TMZ treatment.
DECLARATIONS
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the ethics committee of Peking University Cancer Hospital & Institute. Written consent was obtained from the patient.
CONSENT FOR PUBLICATION
Not Applicable
AVAILABILITY OF DATA AND MATERIALS
Additional data and materials related to the genetic tests, pathologic reports, treatment information, and images are available for review upon reasonable request.
COMPETING INTERESTS
Yutong Ma is an employee of Geneseeq Technology Inc., Canada. Tingting Sun and Yang W. Shao are employees of Nanjing Geneseeq Technology Inc., P. R. China. The remaining authors have no competing interests to declare.
AUTHORS’ CONTRIBUTIONS
ML, JL, and LS contributed to the conception of the work.
YSC contributed to the acquisition and interpretation of data, drafting, and revising the manuscript.
YTM contributed to the analysis of the genetic test results and revision of the manuscript.
JYY contributed to the interpretation of the nuclear medicine results and images.
YS contributed to the interpretation of the pathology results and images.
TTS and YS contributed to the collection of the genetic test results.
ACKNOWLEDGMENTS
We sincerely thank the patient for supporting our work. We thank Drs. Yong Cui, Xiaolei Gu, and Peng Liu for helping to prepare and interpreting the radiology results and images. We also thank Drs. Qiuxiang Ou and Hua Bao for help interpreting the genetic test results.




