Emerging roles of long noncoding RNAs in cholangiocarcinoma: Advances and challenges
Abstract
Cholangiocarcinoma (CCA), a cancer with a relatively low incidence rate, is usually associated with poor prognosis. Current modalities for the diagnosis and treatment of CCA patients are still far from satisfactory. In recent years, numerous long noncoding RNAs (lncRNAs) have been identified as crucial players in the development of various cancers, including CCA. Abnormally expressed lncRNAs in CCA, regulated by some upstream molecules, significantly influence the biological behavior of tumor cells and are involved in tumor development through various mechanisms, including interactions with functional proteins, participation in competing for endogenous RNA (ceRNA) regulatory networks, activation of cancer-related signaling pathways and epigenetic modification of gene expression. Furthermore, several lncRNAs are closely associated with the clinicopathological features of CCA patients, and are promising biomarkers for diagnosing and prognostication of CCA. Some of these lncRNAs play an important role in chemotherapy drug resistance. In addition, lncRNAs have also been shown to be involved in the inflammation microenvironment of CCA and malignant outcome of CCA risk factors, such as cholestatic liver diseases. In view of the difficulty of diagnosing CCA, more attention should be paid to detectable lncRNAs in the serum or bile. This review summarizes the recent knowledge on lncRNAs in CCA and provides a new outlook on the molecular mechanisms of CCA development from the perspective of lncRNAs. Moreover, we also discussed the limitations of the current studies and differential expression of lncRNAs in different types of CCA.
Abbreviations
-
- 5-FU
-
- 5-Fluorouracil
-
- AFAP1
-
- actin filament associated protein 1
-
- AJCC
-
- American Joint Committee on Cancer
-
- AKT
-
- AKT serine/threonine kinase
-
- ANGPTL4
-
- angiopoietin like 4
-
- BAP1
-
- BRCA-1 associated protein-1
-
- BRCA1
-
- Breast cancer 1
-
- CCA
-
- cholangiocarcinoma
-
- CCND1
-
- cyclin D1
-
- CDKN1A
-
- cyclin dependent kinase inhibitor 1A
-
- ceRNA
-
- competing endogenous RNA
-
- circRNA
-
- circular RNA
-
- CPS1
-
- carbamoyl-phosphate synthase 1
-
- CSCs
-
- cancer stem cells
-
- dCCA
-
- distal cholangiocarcinoma
-
- E-cadherin
-
- epithelial cadherin
-
- eCCA
-
- extrahepatic cholangiocarcinoma
-
- ECM
-
- extracellular matrix
-
- EMT
-
- epithelial-to-mesenchymal transition
-
- EVs
-
- extracellular vesicles
-
- EZH2
-
- enhancer of the zeste homologue
-
- FBP1
-
- fructose-bisphosphatase 1
-
- FOXO3
-
- forkhead box O3
-
- GBM
-
- glioblastoma
-
- GLI1
-
- GLI family zinc finger 1
-
- GSK-3β
-
- glycogen synthase kinase 3 beta
-
- HCV
-
- Hepatitis C virus
-
- Hh pathway
-
- Hedgehog pathway
-
- HIF-1α
-
- hypoxia inducible factor-1
-
- HREs
-
- hypoxia response elements
-
- HSCs
-
- hepatic stellate cells
-
- IC50s
-
- the half-maximal inhibitory concentrations
-
- iCCA
-
- intrahepatic cholangiocarcinoma
-
- ID3
-
- inhibitor of differentiation 3
-
- IRF4
-
- interferon regulatory factor 4
-
- lncRNA
-
- long noncoding RNA
-
- MAPK
-
- mitogen-activated protein kinase
-
- miRNA
-
- microRNA
-
- MMP
-
- matrix metalloprotease
-
- N-cadherin
-
- neural cadherin
-
- NF-κB
-
- Nuclear factor-κB
-
- NIK
-
- NF-κB-inducing kinase
-
- OS
-
- overall survival.
-
- PBC
-
- primary biliary cholangitis
-
- pCCA
-
- perihilar cholangiocarcinoma
-
- PCNA
-
- proliferating cell nuclear antigen
-
- PFS
-
- progression-free survival
-
- PI3K
-
- phosphatidylinositol 3-kinase
-
- PRC2
-
- polycomb repressive complex 2
-
- PSC
-
- primary sclerosing cholangitis
-
- RNA-Seq
-
- RNA sequencing
-
- RUNX1
-
- RUNX family transcription factor 1
-
- SETDB1
-
- SET domain bifurcated histone lysine methyltransferase 1
-
- SMOsmoothened
-
- smoothenedfrizzled class receptor
-
- SOX2
-
- SRY-box transcription factor 2
-
- TCGA
-
- the Cancer Genome Atlas
-
- TGF-β
-
- transforming growth factor beta
-
- Thr58
-
- threonine 58
-
- TLR
-
- Toll-like receptor
-
- Wnt
-
- wingless-type MMTV integration site family
-
- α-SMA
-
- α-smooth muscle actin
1 INTRODUCTION
With the widespread application of microarray and high-throughput sequencing technology in studies of the whole genome and transcriptome, it is now clear that while less than 2% of the genome encodes proteins, at least 75% of the genome is actively transcribed into noncoding RNAs; most of which are longer than 200 nucleotides, and are classified as long noncoding RNAs (lncRNAs). Initially, lncRNAs were regarded as transcriptional noise of genes due to their lacking ability to encode proteins. Nevertheless, in recent years, growing evidence have shown that lncRNAs have several distinctive features including exquisite cell- and tissue-specific expression, and the capacity to regulate gene expression at different levels [1, 2]. Moreover, the dysregulation of lncRNAs was demonstrated to have strong relevance not only in neurological, cardiovascular, and developmental diseases but also in tumorigenesis [3]. Prostate cancer-associated 3 (PCA3), the first lncRNA identified to be abnormally expressed in cancer, is already being used as a biomarker in prostate cancer for clinical diagnosis [4]. Along with increasing publications regarding the mechanism of lncRNAs, the roles of lncRNAs in cancer are constantly being clarified. LncRNAs can serve as transcriptional targets of factors that control cellular homeostasis such as the tumor suppressor P53 and oncogene MYC [5, 6]. Furthermore, studies have indicated that diverse lncRNAs are involved in the splicing, export, and translation of mRNAs, as well as in the stability and post-translational modification of proteins. Additionally, lncRNAs were demonstrated to act as competing endogenous RNAs (ceRNAs), interacting with microRNAs (miRNAs) in a way that can segregate these molecules and attenuate their regulatory effect on target mRNAs [7].
Cholangiocarcinoma (CCA) is a disease entity composed of a variety of epithelial tumors that are characterized by cholangiocyte differentiation. Depending on their anatomical location, cholangiocarcinoma can be categorized as intrahepatic cholangiocarcinoma (iCCA), perihilar cholangiocarcinoma (pCCA), and distal cholangiocarcinoma (dCCA); with the latter two collectively referred to as extrahepatic cholangiocarcinoma (eCCA) [8]. Over the past several decades, the incidence rate of CCA has increased worldwide. From 1999 to 2013, the iCCA (estimated annual percent change of 3.2%/year; P < 0.0001) and eCCA rates (1.8%/year; P = 0.001) have steadily increased across sex and racial/ethnic groups in the United States [9]. Since 2000, the incidence rate of iCCA in Southeast Asian countries, especially Thailand, has been rising sharply, and this growth is estimated to last until 2025 [10]. Recently, cirrhosis and viral hepatitis C and B have been recognized as risk factors for cholangiocarcinoma. Additionally, primary sclerosing cholangitis (PSC) is tightly associated with cholangiocarcinoma, and 50% of patients with primary sclerosing cholangitis are diagnosed with cholangiocarcinoma within 1 year of being diagnosed with primary sclerosing cholangitis. Due to the high prevalence of hepatobiliary flukes, i.e. Clonorchis Sinensis and Opisthorchis viverrini, Southeast Asia has a very high incidence (113 per 100 000) of cholangiocarcinoma [11].
Further, due to the lack of specific symptoms and sensitive diagnostic indicators, most CCA patients are diagnosed at a late stage. At present, surgical resection is considered as the only potentially effective therapy, with a 5-year overall survival rate ranging between 15% and 40%. However, the recurrence rate after resection can be as high as 65% [12]. Currently, the serum levels of CA19-9 are widely used in the clinical diagnosis of CCA, but the sensitivity and specificity are quite low, at only ∼62% and ∼63%, respectively [13]. Therefore, it is extremely necessary to discern the molecular mechanisms of CCA, explore new tumor biomarkers, and discover effective diagnostic and therapeutic targets of CCA. In comparison to the knowledge in cancers with a high incidence rate, the roles of lncRNAs in cholangiocarcinoma are not fully understood. Here, we reviewed the recent studies on lncRNA involvement and mechanisms in the development of CCA. We also summarized the upstream regulators of lncRNAs and their roles in the inflammatory microenvironment and risk factors of CCA, to provide a new perspective for understanding CCA from the viewpoint of lncRNAs. Additionally, we discussed the possibility of lncRNAs applications in the diagnosis and treatment of CCA patients. It is undeniable that the lacking of in-depth mechanisms and large sample sizes are becoming hindrances to the lncRNA studies in CCA.
2 THE ROLE OF lncRNAs IN CHOLESTATIC LIVER DISEASES
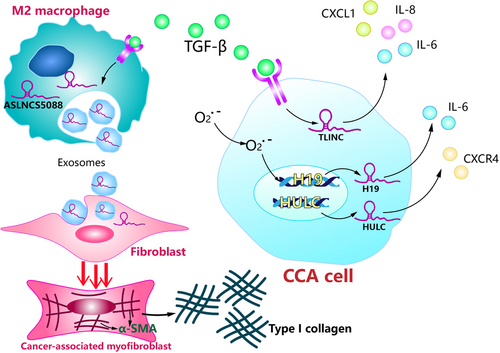
In cholestatic liver diseases, including primary sclerosing cholangitis (PSC) and primary biliary cholangitis (PBC), the obstruction of bile flow can lead to bile acid accumulation, which not only results in hepatocyte injury and chronic inflammation but also induces liver fibrosis and malignant proliferation of cholangiocytes. The activation of hepatic stellate cells (HSCs) plays an essential role in the progression of liver fibrosis in chronic cholestatic liver diseases [14]. Exosomes are small, single-membrane, secreted organelles with a diameter of 30 nm-200 nm that can remodel the extracellular matrix, and transmit signals and molecules to other cells [15]. Liu et al [16] discovered that hepatic lncRNA H19 levels were positively correlated with the severity of liver fibrosis in both cholestatic mouse models and human patients with PSC and PBC. Under cholestatic conditions, HSCs preferentially ingest cholangiocyte-derived exosomes. Subsequently, overexpressed exosomal H19 accelerated HSC activation and proliferation, and thus promoted liver fibrosis.
Fate-mapping studies have revealed that iCCA may originate from the transdifferentiation of hepatocytes, and the transdifferentiation of hepatocytes into cholangiocytes is also observed in chronic liver injury [14]. Li et al [17] demonstrated that the hepatic lncRNA H19 level was also correlated with the severity of cholestatic injury in both fibrotic mouse models and human PSC patients. Cholangiocyte-derived exosomes can mediate the transfer of H19 into hepatocytes. Then, H19-carrying exosomes suppress small heterodimer partner expression in hepatocytes to promote cholestatic liver injury.
3 ABERRANTLY EXPRESSED PROFILES OF lncRNAs IN CCA
With the extensive application of RNA sequencing (RNA-Seq) data from the Cancer Genome Atlas (TCGA) database and bioinformatics technology, a large number of lncRNAs differentially expressed in CCA are being identified at a phenomenal rate. Wang et al. [18] identified a total of 1434 differentially expressed lncRNAs between CCA tissues and normal tissues, of which 929 were upregulated and 505 were downregulated. In the study of Wang et al. [19], a total of 2773 lncRNAs were significantly upregulated in iCCA tissues compared with noncancerous tissues, whereas 2392 lncRNAs were downregulated. Nevertheless, some studies were limited to a relatively small sample size, and have not elaborated on the specific type of CCA samples. Interestingly, there were significant differences in the expression of lncRNA SPRY4-IT1 in cell lines of different types of CCA. More specifically, SPRY4-IT1 was found to be highly expressed in iCCA cell lines but not in pCCA and dCCA cell lines [20]. In a collaborative effort by the International Cancer Genome Consortium, an integrative cluster analysis based on 489 CCA samples from 10 different regions identified 4 subgroups, each possessing distinct genetic, epigenetic, and clinicopathological characteristics [21]. This taxonomy can contribute to the discovery of new potential CCA driven genes (RASA1, STK11, MAP2K4, and SF3B1) and gene rearrangement (FGFR2 3’ UTR deletion) [21]. Given the considerable genetic diversity of CCA, it might be more reasonable and effective to carry out explorations of lncRNA expression profiles based on molecular clustering.
4 CCA-SPECIFIC INFLAMMATORY MICROENVIRONMENT
TP73-AS1, which is upregulated in CCA cells, does not affect the growth and apoptosis of normal human intrahepatic bile duct epithelial cells [22]. In addition, high expression of LINC00261 was shown to promote metastasis through the EMT process in CCA [23], while downregulated LINC00261 was found to be associated with poor prognosis in digestive system malignancies, including gastric cancer [24], hepatocellular carcinoma [25], and pancreatic cancer [26]. These phenomena suggest that there might be a tumor-specific differential expression pattern of lncRNAs in the CCA microenvironment.
Active TGF-β signaling is regarded as a significant feature in the iCCA microenvironment [27]. Merdrignac et al [28] identified a novel transcriptional target of TGF-β, LINC00340 (TLINC), in iCCA. TLINC can produce multiple transcripts by alternative splicing, including a short (TLINC-S) and a long (TLINC-L) isoform. A subsequent study showed that TLINC-L and TLINC-S were induced by TGF-β in two different CCA cell lines, respectively, HuCCT1 and Huh28, indicating that the function of TLINC could be highly dependent on the cellular context [28]. After the overexpression of TLINC-L, some inflammation-associated genes (e.g.: IL6, IL8, and CXCL1) were induced to be overexpressed. IL8 has been shown to be abnormally overexpressed in cancer and functions as a significant regulatory factor within the tumor microenvironment of processes including angiogenesis, migration, tumorigenicity, and infiltration of neutrophils and tumor-associated macrophages [28].
Cholangiocytes under oxidative stress might induce and reinforce inflammatory responses. The malignant transformation of cholangiocytes is thought to be associated with chronic inflammation in the biliary epithelium [29]. After both short- and long-term oxidative stress, lncRNAs HULC, and H19 were found upregulated in CCA cell lines and significantly promoted the migration and invasion of CCA cells. Further investigations showed that H19 and HULC could regulate the migration and invasion of CCA cells by enhancing IL-6 and CXCR4 levels via sponging let-7a/let-7b and miR-372/miR-373, respectively [30]. IL-6 is a multifunctional inflammatory cytokine that is believed to play a significant role in the inflammatory response of cholangiocytes. Increased concentrations of IL-6 in the inflammatory microenvironment of the biliary tract could stimulate several oncogenic signaling pathways such as the ERK, JAK-STAT, and PI3K pathway [31]. CXCR4 is a chemokine receptor that is also involved in the inflammatory microenvironment of CCA, and can promote CCA cell migration and invasion through the Akt pathway [32].
Han et al. [33] further examined the expression patterns of adjacent genes and co-expressed genes of lncRNAs in CCA. They found that 184 of the 583 lncRNAs abnormally expressed in CCA tissue were located near inflammation-related genes, and some were located in the same genomic region, such as lncRNA XLOC_004959 and CTC-505O3.2, which are members of the Toll-like receptor signaling pathway, were adjacent to TICAM2 and TMED7-TICAM2. Interestingly, another set of lncRNAs, APOC1P1, LPAL2, and RP11-370B11.3 were identified to be co-expressed with inflammatory factors without neighboring inflammatory genes, indicating that these lncRNAs might regulate inflammatory pathways in a non-cis manner [33].
Cholangiocarcinoma typically exhibits a prominent desmoplastic reaction characterized by the formation of dense type I collagen fiber-enriched tumor stroma, and the presence of α-smooth muscle actin-positive cancer-associated myofibroblasts, which positively correlates with poorer survival outcomes in CCA patients after surgical resection. Chen et al. [34] demonstrated that under the stimulation of TGF-β1, lncRNA ASLNCS5088, which was enriched in M2 macrophage-derived exosomes, could be efficiently transferred into fibroblasts, resulting in increased α-SMA expression and activation of myofibroblasts. In contrast, MEG3, a well-studied lncRNA with tumor-suppressive effects in CCA, was found to be reduced in vivo and in vitro during liver fibrosis. Restoring MEG3 expression resulted in the suppression of liver fibrosis, with a reduction in α-SMA and type I collagen [35]. Therefore, it is reasonable to assume that lncRNAs play a crucial role in the progression of liver fibrosis to cholangiocarcinoma (Figure 1).
5 REGULATORS OF lncRNAs IN CCA
Several transcription factors have been reported to induce the expression of lncRNAs in CCA. E2F1, as a member of the classical transcription factor family, is implicated in the regulation of many cellular processes in cancer, including proliferation and apoptosis [36]. Two E2F1 response elements (E1 and E3) are functional and indispensable for the transcriptional activation of the lncRNA LMCD1-AS1 promoter in CCA, while E2 is not [37]. Interferon regulatory factor 4 (IRF4) is a transcription factor that plays a crucial role in the differentiation of B cells and is widely involved in malignancies of the hematological system [38]. IRF4 can bind to part 2 (P2) of the SOX2-OT promoter and positively modulate the expression level of SOX2-OT in CCA cell lines [39]. In addition, the transcription factor SP1 can bind directly to the SPRY4-IT1 promoter region and facilitate SPRY4-IT1 expression in CCA [20].
Hypoxia is a common feature of the tumor microenvironment which originates from the imbalance of oxygen supply and consumption in fast-growing tumors. Hypoxia-inducible factor-1 alpha (HIF-1α) is a key transcriptional regulator in response to cellular hypoxia [40]. HIF-1α can directly interact with specific sites at hypoxia response elements (HREs) in the lncRNA promoter region, and regulate the expression of lncRNAs [41]. This mechanism has been confirmed in multiple gastrointestinal cancers but not in cholangiocarcinoma [42]. However, lncRNAs such as PVT1 [43], UCA1 [44], and ANRIL [45], which have been verified to be induced by hypoxia in gastrointestinal cancers [41, 42], were also abnormally expressed in CCA, suggesting that further exploration of hypoxia-regulated lncRNAs in CCA should be carried out.
In most studies on cancer, TGF-β is generally considered as the downstream molecule of abnormally expressed lncRNAs. For instance, overexpressed lnc-LFAR1 can accelerate the EMT process by regulating the TGF-β/Smad pathway to promote the development of CCA [46] As mentioned above, LINC00340 (TLINC) is induced by TGF-β in CCA cell lines, and the induction can be abolished in the presence of a TGF-β pathway inhibitor [28]. The TGF-β pathway involves diverse molecules in cancer but it is not clear which mechanism mediates the overexpression of lncRNAs in cholangiocarcinoma.
Although there are a large number of aberrantly expressed lncRNAs in CCA, the causative factors of their abnormal expression remain unclear. In addition to several classical transcription factors and HIF-1α, TGF-β plays a significant role in inducing abnormal expression of lncRNAs in CCA on account of a well-established association between chronic inflammation and CCA. Future studies should pay more attention to the lncRNA-mediated regulation of inflammatory factors in CCA. Additionally, lncRNA epigenetic landscape analysis has attributed the overexpression of EPIC1 to recurrent hypomethylation of its host gene across 6475 tumors and 455 cancer cell lines [47]. Therefore, research on epigenetic modification of genes encoding lncRNAs would enhance our understanding on the origins of abnormal lncRNA expression in CCA.
6 EFFECTS OF LNCRNAS ON THE BIOLOGICAL CHARACTERISTICS OF CCA CELLS
6.1 Promotion of the migration and invasion of CCA cells
Studies from different groups have confirmed that the dysregulation of lncRNA expression is closely related to the biological behavior of CCA cells. Upregulation of these lncRNAs, including KCNQ1OT1 [48], LMCD1-AS1 [37], TP73-AS1 [22], SNHG1 [49], SOX2-OT [39], LINC01410 [50], ASAP1-IT1 [51], DANCR [52], FLVCR1-AS1 [53], NNT-AS1 [54], RMRP [55], CCAT2 [56], HOTAIR [57], LINC01296 [58], LOXL1-AS1 [59], NEAT1 [60], PVT1 [43], HEIH [61], LINC01061 [62], may promote the migration and invasion of CCA cells, . On the other hand, the downregulation of several lncRNAs plays the same role in CCA cells including LINC01714 [63], FENDRR [64], MEG3 [65], and MIR22HG [66]. Similarly, in studies of lncRNAs in iCCA, NEF [67], LFAR1 [46], SNHG3 [68], UCA1 [44], CRNDE [69] and CCAT2 [70], and can also facilitate the migration and invasion of iCCA cells (Table 1).
| LncRNA | Chromosomal location | Expression | Cell/Tissue | Role | Target gene | Mechanism | Function | Reference |
|---|---|---|---|---|---|---|---|---|
| LNCNEF | 20p11.21 | down | cell, tissue | tumor suppressor | RUNX1(↑) | - | inhibition of EMT, cell migration and invasion | [67] |
| TP73-AS1 | 1p36.32 | up | cell, tissue | oncogene | - | - | promotion of cell proliferation, migration, invasion, anti-apoptosis, and tumorigenesis in vivo | [22] |
| SNHG1 | 11q12.3 | up | cell, tissue | oncogene | CDKN1A(↓) | EZH2-mediated H3K27me3 demethylation | promotion of cell proliferation, migration, invasion, anti-apoptosis, and tumorigenesis in vivo | [49] |
| SOX2-OT | 3q26.33 | up | cell, tissue | - | SOX2(↑) | activation of PI3K/AKT signaling pathway | promotion of cell proliferation, migration, invasion, and anti-apoptosis | [39] |
| LINC01714 | 1p36.23 | down | cell, tissue | tumor suppressor | FOXO3(↓) | interaction with FOXO3 protein, inhibition of the phosphorylation status of FOXO3 protein | inhibition of cell proliferation, migration, invasion, and tumorigenesis in vivo | [63] |
| LFAR1 | 4q25 | up | cell | - | - | activation of TGF-β/Smad signaling pathway | promotion of EMT, cell proliferation, migration, and invasion | [46] |
| lnc-PKD2-2-3 | 4q21 | up | cell, tissue | CSC marker | - | - | promotion of cell stemness | [87] |
| ASAP1-IT1 | 8q24.21 | up | cell, tissue | - | Smo, Gli1(↑) | activation of TGF-β/Smad signaling pathway | promotion of EMT, cell proliferation, migration | [51] |
| DANCR | 4q12 | up | cell, tissue | oncogene | FBP1(↓) | EZH2-mediated H3K27me3 demethylation | promotion of cell proliferation, migration, anti-apoptosis, and tumorigenesis in vivo | [52] |
| FENDRR | 16q24.1 | down | cell, tissue | - | survivin(↑) | SETDB1-mediated H3K9 methylation | inhibition of cell proliferation, migration, and invasion | [64] |
| MEG3 | 14q32.2 | down | cell, tissue | tumor suppressor | Bmi1, RNF2(↑) | - | inhibition of EMT, cell proliferation, migration, invasion, and tumorigenesis in vivo | [65] |
| ANRIL | 9p21.3 | up | cell, tissue | oncogene | ERRFI1(↓) | EZH2-mediated H3K27me3 demethylation | promotion of cell proliferation, migration, anti-apoptosis, and tumorigenesis in vivo, regulation of cell cycle progression | [45] |
| SNHG3 | 1p35.3 | up | tissue | - | - | - | [68] | |
| CCAT2 | 8q24.21 | up | cell, tissue | - | - | - | promotion of EMT, cell proliferation, migration, invasion, and anti-apoptosis | [70] |
| EPIC1 | 22q13.31 | up | cell, tissue | - | MYC(↑) | interaction with MYC protein | anti-apoptosis and regulation of cell cycle progression | [90] |
| HOTAIR | 12q13.13 | up | cell, tissue | oncogene | - | - | promotion of EMT, cell proliferation, migration, invasion, anti-apoptosis, and tumorigenesis in vivo | [57] |
| MIR22HG | 17p13.3 | down | cell, tissue | tumor suppressor | - | inactivation of Wnt/β-catenin signaling pathway | inhibition of cell proliferation, migration, invasion, and tumorigenesis in vivo | [66] |
| NEAT1 | 11q13.1 | up | cell, tissue | oncogene | E-cadherin(↓) | EZH2-mediated H3K27me3 demethylation | promotion of cell proliferation, migration, invasion, and tumorigenesis in vivo | [60] |
| PVT1 | 8q24.21 | up | cell, tissue | oncogene | ANGPTL4(↓) | EZH2-mediated H3K27me3 demethylation | promotion of cell proliferation, migration, invasion anti-apoptosis, and tumorigenesis in vivo | [43] |
| UCA1 | 19p13.12 | up | cell, tissue | oncogene | - | activation of AKT/GSK-3β/CCND1 signaling pathway | promotion of EMT, cell proliferation, migration, invasion, and anti-apoptosis | [83] |
| CRNDE | 16q12.2 | up | cell, tissue | - | - | - | promotion of EMT, cell proliferation, migration, invasion | [69] |
| LINC00261 | 20p11.21 | up | cell, tissue | - | - | - | promotion of EMT, cell proliferation, migration, invasion, and anti-apoptosis | [23] |
| H19 | 11p15.5 | up | cell, tissue | - | - | - | promotion of EMT, cell proliferation, migration, invasion, and anti-apoptosis | [179] |
| CPS1-IT1 | 2q34 | up | cell, tissue | - | - | co-expression with CPS1 | promotion of cell proliferation and anti-apoptosis | [120] |
| AFAP1-AS1 | 4p16.1 | up | cell, tissue | - | AFAP1(↓) | - | promotion of cell proliferation, migration, invasion, and anti-apoptosis | [118] |
| MALAT1 | 11q13.1 | up | cell | - | - | activation of PI3K/AKT signaling pathway | promotion of EMT, cell proliferation, migration, invasion | [130] |
| PANDAR | 6p21.2 | up | cell, tissue | - | - | - | promotion of EMT, cell proliferation, migration, invasion, and anti-apoptosis | [74] |
- Abbreviations: CCA, cholangiocarcinoma; lncRNA, long noncoding RNA; RUNX1, RUNX family transcription factor 1; EMT, epithelial-to-mesenchymal transition; CDKN1A, cyclin-dependent kinase inhibitor 1A; SOX2, SRY-box transcription factor 2; SMO, smoothened, frizzled class receptor; GLI1, GLI family zinc finger 1; FBP1, fructose-bisphosphatase 1; BMI1, BMI1 proto-oncogene, polycomb ring finger; RNF2, ring finger protein 2; ERRFI1, ERBB receptor feedback inhibitor 1; ANGPTL4, angiopoietin-like 4; AFAP1, actin filament associated protein 1; EZH2, enhancer of the zeste homolog; PI3K, phosphatidylinositol 3-kinase; AKT, AKT serine/threonine kinase; FOXO3, forkhead box O3; TGF-β, transforming growth factor-beta; SETDB1, SET domain bifurcated histone lysine methyltransferase 1; MYC, MYC proto-oncogene; Wnt, wingless-type MMTV integration site family; GSK-3β, glycogen synthase kinase 3 beta; CCND1, cyclin D1; CPS1, carbamoyl-phosphate synthase 1.
Epithelial-to-mesenchymal transition (EMT) is a fundamental process regulating the transformation of cells from an epithelial to a mesenchymal state. Primary epithelial tumor cells are transformed into mesenchymal cells with migration ability through the third type of EMT which governs tumor invasiveness, metastasis, and prognosis. During EMT, epithelial cadherin (E-cadherin) and claudins are downregulated while neural cadherin (N-cadherin), vimentin, and fibronectin are upregulated [71]. The E/N-cadherin switch has been identified as an essential step in manipulating cell invasion and metastasis in CCA. Zhang et al [60] found that the knockdown of NEAT1 significantly downregulated the levels of Vimentin and N-cadherin but increased E-cadherin expression in CCA cells. NEAT1 suppresses the expression of E-cadherin partly by binding EZH2, enabling hypermethylation of the E-cadherin promoter. Snail is a major transcription factor governing EMT, and snail-induced EMT has been validated to accelerate cancer metastasis through the induction of immunosuppression [72]. Silencing SPRY4-IT1 in CCA cells can increase the expression levels of E-cadherin and decrease the expression levels of Snail and vimentin [20]. The matrix metalloprotease (MMP) family is capable of activating TGF-β by inducing proteolysis to promote EMT, and MMP-9 has been confirmed to enhance the invasion capacity of cancer cells by damaging the histological barrier around the extracellular matrix [73]. Experiments have proved that the overexpression of the lncRNAs NNT-AS1 and PANDAR in CCA could enhance the expression of N-cadherin, MMP2 and MMP9, and decreased the expression of E-cadherin [54, 74].
Oxygen and nutrients supplied by the vasculature are essential for the function and survival of aberrantly proliferating cancer cells. To expand malignant lesions, incipient neoplasms tend to develop angiogenic ability. Moreover, angiogenesis can assist cancer cells in obtaining invasion and migration abilities. The E2F8 mutantin in extraembryonic trophoblastic cells is a proven key factor involved in the structural abnormalities of the placental vascular network [75]. Wang et al [76] discovered that medium derived from lncRNA SNHG6 silenced CCA cells could suppress Human Umbilical Vein Endothelial Cells (HUVECs) tube formation, and SNHG6 exerted its biological role mainly by targeting E2F8. Therefore, these suggest that lncRNAs may have an important role in the metastasis of cholangiocarcinoma cells by regulating angiogenesis.
Current studies have shown that the aggressive behaviors of CCA are regulated by lncRNAs and can be reflected in the high invasiveness and migration of CCA cells, activation of EMT, and angiogenesis. Additionally, the extracellular matrix (ECM) component of solid tumors has been implicated in metastatic progression. Altered macromolecular substrate activity in the ECM of cancer triggers pathological ECM remodeling and facilitates metastatic dissemination [77]. It will be a novel approach to explore the regulation of CCA cell migration and invasion by lncRNAs from the perspective of ECM because compared with the direct study of cell lines, it could better simulate the in vivo tumor microenvironment.
6.2 Proliferation and anti-apoptosis effects on CCA cells
Unrestricted proliferation is one of the most fundamental characteristics of cancer cells. Most of the abnormally expressed lncRNAs in cholangiocarcinoma have been proven to be closely related to cell proliferation. (Table 1) For example, lncRNA TUG1 has been identified to be upregulated in CCA. When TUG1 was depleted, the protein level of PCNA in CCA cells was downregulated, followed by the inhibition of cell proliferation [78]. Proliferating cell nuclear antigen (PCNA) is widely known as an important factor in the metabolism of nucleic acids and plays a critical role in many aspects of DNA replication [79]. In addition, lncRNA FENDRR can inhibit the proliferation of CCA cells through the suppression of survivin [64]. Survivin is an evolutionarily conserved protein that is essential for cell division, and is generally expressed only in actively proliferating cells [80].
Cancer is regarded as a scenario where little apoptosis occurs, leading to an abundance of long-living malignant cells [81]. Some of the upregulated lncRNAs mentioned above are also closely related to the inhibition of CCA cell apoptosis, while overexpression of those downregulated lncRNAs can accelerate apoptosis. Among the 14 mammalian caspases discovered so far, caspase-3 and caspase-9 play a key role in the regulation of apoptosis. Additionally, Bcl-2 family proteins are regarded as central regulators of programmed cell death [82]. LncRNA UCA1 acts as an anti-apoptotic factor in CCA, and apoptosis-associated factors (caspase-3, and -9) are both increased followed by the knockdown of UCA1. Subsequent studies also confirmed that downregulated UCA1 increased apoptosis by activating the expression of Bax and suppressing Bcl-2 expression [83].
Attenuated apoptosis and unrestricted proliferation are fundamental characteristics of cancer cells. Numerous lncRNAs have been proven to regulate the expression of classic proliferation- and apoptosis-related proteins, thus participating in the biological characteristics of CCA cells.
6.3 Enhancement of CCA cells stemness
Genomic instability and the unique microenvironment of tumors have contributed to the discovery of small subpopulations of cancer cells with a high capacity for self-renewal and differentiation, and the ability to initiate tumorigenesis, which are referred to as cancer stem cells (CSCs) [84]. Emerging evidence indicates that lncRNAs are key positive regulators of the CSC subpopulation and contribute to self-renewal, drug resistance, and EMT in diverse cancers [85]. Wang et al [86] found that lncTCF7 activates TCF7 expression locally (in cis) by recruiting the SWI/SNF complex, an evolutionally conserved multi-subunit complex that can regulate gene expression, and TCF7 expression induces the Wnt signaling pathway to initiate self-renewal of the liver CSCs. Overexpression of lnc-PKD2-2-3 in CCA cells significantly increased CSC marker expression (CD44, CD133, and OCT4) and improved sphere formation efficiency, indicating that lnc-PKD2-2-3 may promote CCA stemness and potentially serve as a marker for CSCs in CCA [87]. In addition, lncRNA RMRP is highly expressed in CCA, and cancer stem cell-related markers Bmi1 and CD24 were found to be significantly decreased after RMRP knockdown, but surprisingly, the expression of CD44 remained unchanged [55]. There is a need for additional research on the role of RMRP as a CCA cell stemness promoter. However, this sole study in CCA did not determine the role of lncRNAs in cholangiocyte CSC self-renewal.
Inhibitor of differentiation 3 (ID3), which is highly expressed in human iCCA tissues compared with matched normal tissues, was found essential for stemness maintenance in iCCA and could serve as a promising biomarker in predicting iCCA patient responses to adjuvant chemotherapeutics [88]. Interestingly, Sanzo et al [89] demonstrated that the expression level of ID3 was regulated by lncRNA H19 in a leukemia cell line. Cancer stem cells (CSCs) tend to be responsible for chemoresistance. As a result, probing the role of lncRNAs in CSCs will contribute to possible therapeutic strategies.
7 MECHANISMS OF lncRNAs PROMOTING CCA DEVELOPMENT
7.1 Direct interaction with functional proteins
In breast cancer cells, lncRNA EPIC1 was identified to directly interact with the 148-220 aa region of the MYC protein through its 129-283 nt sequence, and then specifically regulate the occupancy of MYC on target genes to promote breast cancer [47]. Li et al [90] confirmed that EPIC1 was highly expressed in CCA tissues and plays an oncogenic role during CCA development by directly targeting the MYC protein.
Phosphorylation of the threonine 58 (Thr58) site on MYC protein resulted in its degradation through the ubiquitin-proteasome pathway. In human breast cancer cells, lncRNA PVCT1 can increase the level of MYC protein by blocking the phosphorylation of the Thr58 site on MYC [91]. In the human genome, MYC is located only 53 kb upstream of PVT1, and both of them have been reported to play important roles in CCA [43, 92]. Therefore, whether there is a direct interaction between MYC and PVT1 in CCA remains to be confirmed in subsequent studies.
LINC01714, which is downregulated in CCA cells, was found to interact with the forkhead (FH) DNA-binding domain of the FOXO3 protein through its 1- to 195-nt fragment. Moreover, the overexpression of LINC01714 significantly decreased the phosphorylation level of FOXO3-Ser318, and in comparison to CCA patients with low LINC01714-FOXO3 levels, those with high LINC01714-FOXO3 levels had better overall survival [63].
Both MYC and FOXO3 are widely-studied functional proteins in cancer. However, it is still unclear which domains of MYC can directly interact with those of EPIC1 and whether EPIC1 is involved in MYC occupancy on target genes in CCA.
7.2 ceRNA regulatory network
In 2011, Salmena et al [93] first proposed the well-known competing endogenous RNA (ceRNA) hypothesis, which states that RNAs can cross-talk with each other and manipulate biological functions independently of protein translation. Studies have demonstrated that lncRNAs can function as ceRNAs to compete with mRNAs for binding to miRNAs like sponges and further regulate gene expression [94]. Furthermore, this mechanism has been widely demonstrated to play an important role in the carcinogenesis and development of numerous cancers, including cholangiocarcinoma (Figure 2).

miR-203, a well-described miRNA, has been shown to play a critical role in tumor development [95]. Gu et al [96] found that lncRNA NNT-AS1 could sponge miR-203 and downregulate the expression of miR-203 in CCA cells. The proliferation and EMT process of CCA cells were notably accelerated by NNT-AS1 overexpression, but after the miR-203 mimic was transfected into CCA cells, both proliferation and EMT were inhibited. This study also corroborated that NNT-AS1 overexpression promoted the activation of the PI3K/AKT and ERK1/2 signaling pathways by downregulating miR-203. However, whether the interaction between miR-203 and ZEB1/IGF1R, two potential candidate targets of miR-203, is involved in the cancer-promoting effect of NNT-AS1 remains to be further confirmed [96]. In addition, lncRNA SNHG1, as a ceRNA for miR-140, can promote TLR4 expression and activate the NF-κB signaling pathway, thus regulating the growth and tumorigenesis of CCA [97]. LncRNA UCA1 was confirmed to directly bind to miR-122 and negatively regulate the expression of miR-122 in iCCA cells. In addition, miR-122 mimics inhibited the promoting effects of UCA1 on iCCA cell proliferation and invasion [44]. In another study, UCA1 was considered to be an activator of the AKT/GSK3β/CCND1 signaling pathway in CCA [83]. Therefore, whether miR-122 is involved in the activation of this pathway remains to be explored.
Currently, cancer is considered a special metabolic disease, and abnormal glutamine metabolism has proven to be a critical hallmark of cancer by participating in carcinogenesis [98]. Sirt3, a member of the Sirtuin family, was identified as a positive regulator of glutamate dehydrogenase (GDH). The upregulated lncRNA TUG1 significantly increased Sirt3 and GDH expression by sponging miR-145 in iCCA cells, resulting in the enhancement of glutamine metabolism [99]. In cholangiocarcinoma, in addition to miR-203, lncRNA NNT-AS1 could also sponge miR-142-5P and miR-485, and then target HMGA2 and BCL9, respectively, to play a tumor-promoting role; suggesting that there may be a complex ceRNA regulatory network in the development of CCA [54, 100].
Researchers from Harbin Medical University and Miami University jointly constructed a comprehensive database LnCeVar (http://www.bio-bigdata.net/LnCeVar/) aimed at providing a lncRNA-associated ceRNA regulatory network for investigating the functions and mechanisms of personalized genomic variations. This database is based on the published literature and high-throughput datasets including TCGA, COSMIC, and 1000 Genomes Project, and demonstrates the potential for the generation of biomarkers that may be conducive to a better understanding of individual disease pathology [101]. Zhang et al [102] obtained RNA-sequencing datasets of lncRNAs, miRNAs, and mRNAs in CCA and relevant clinical information from The Cancer Genome Atlas (TCGA) database. After that, differentially expressed RNAs were identified and selected to construct a ceRNA network that contained 28 molecules and 47 interactions. In this ceRNA network, seven lncRNAs were identified and expected to become new research targets. However, the authors only utilized a small sample size (36 tumor samples and 9 non-tumor samples) which could generate false-positive findings. In another related study, Xu et al [103] constructed an iCCA-related ceRNA regulatory network that included 340 lncRNA-miRNA-mRNA regulatory relationships based on the NCBI GEO database. Next, a core regulatory pathway RP11-328K4.1-hsa-miR-27a-3p-PROS1 was identified and subsequently validated to be a tumor suppressor pathway in iCCA.
With the application of specific biochemical and computational approaches, a large number of circular RNAs (circRNAs) have been identified in eukaryotes. CircRNAs are covalently closed noncoding RNA molecules generated by a process named back-splicing, and are implicated in various diseases, including cancer, by acting as microRNA or protein sponges [104]. However, few studies have utilized circRNA-containing whole-transcriptome sequencing technology, which allows accurate examination of the transcriptomic landscape of CCA. Chu et al [105] explored the molecular mechanisms of CCA by performing circRNA-containing whole-transcriptome sequencing and validation with the TCGA database. In this study, miR-144-3p was identified to be located in the center of the ceRNA network and might play an important role in the pathogenesis of CCA. Future mechanistic studies should determine the role of miR-144-3p in CCA and which noncoding RNAs function as sponges. According to the latest studies, we summarized some of the ceRNA regulatory pathways in Table 2.
| LncRNA | miRNA(miRNA sponges) | mRNA(competing mRNAs) | Functions | Reference |
|---|---|---|---|---|
| HEIH | miR-98-5p | HECTD4 | proliferation, migration, invasion | [61] |
| KCNQ1OT1 | miR-140-5p | SOX4 | proliferation, anti-apoptosis, invasion, EMT | [48] |
| LINC01061 | miR-612 | SEMA4D | proliferation, anti-apoptosis, migration | [62] |
| LINC01296 | miR-5095 | MYCN | proliferation, anti-apoptosis, migration, invasion | [58] |
| LINC01410 | miR-124-3p | SMAD5 | proliferation, migration, EMT | [50] |
| LMCD1-AS1 | miR-345-5p | COL6A3 | proliferation, anti-apoptosis, invasion | [37] |
| LOXL1-AS1 | miR-324-3p | ABCA1 | proliferation, anti-apoptosis, migration, invasion | [59] |
| MEG3 | miR-361-5p | TRAF3 | proliferation, anti-apoptosis, NF-κB signaling pathway | [147] |
| NNT-AS1 | miR-142-5p | HMGA2 | proliferation, migration, invasion, EMT | [54] |
| miR-203 | ZEB1, IGF1R* | proliferation, EMT, PI3K/AKT, and ERK1/2 pathways | [96] | |
| miR-485 | BCL9 | proliferation, migration, invasion | [100] | |
| SNHG1 | miR-140 | TLR4 | proliferation, invasion, NF-κB signaling pathway | [97] |
| SNHG6 | miR-101-3p | E2F8 | proliferation, angiogenesis | [76] |
| SPRY4-IT1 | miR-101-3p | EZH2 | proliferation, migration, invasion, anti-apoptosis, EMT | [20] |
| MALAT1 | miR-204 | CXCR4 | proliferation, migration, and invasion | [180] |
| PCAT1 | miR-122 | WNT1 | proliferation, migration, anti-apoptosis, Wnt/b-catenin signaling pathway | [135] |
| MIAT | miR-551b-3p | CCND1 | proliferation, anti-apoptosis | [181] |
- Note: * Whether the interaction between miR-203 and ZEB1/IGF1R is involved in the cancer-promoting effect of NNT-AS1 remains to be further confirmed
- Abbreviations: CCA, cholangiocarcinoma; ceRNA, competing endogenous RNA; lncRNA, long noncoding RNA; miRNA, mircoRNA; HECTD4, HECT domain E3 ubiquitin-protein ligase 4; SOX4, SRY-box transcription factor 4; EMT, epithelial-to-mesenchymal transition; SEMA4D, semaphorin 4D; MYCN, MYCN proto-oncogene, bHLH transcription factor; SMAD5, SMAD family member 5; COL6A3, collagen type VI alpha 3 chain; ABCA1, ATP binding cassette subfamily A member 1; TRAF3, TNF receptor associated factor 3; HMGA2, high mobility group AT-hook 2; ZEB1, zinc finger E-box binding homeobox 1; IGF1R, insulin like growth factor 1 receptor; BCL9, BCL9 transcription coactivator; TLR4, toll like receptor 4; E2F8, E2F transcription factor 8; EZH2, enhancer of zeste 2 polycomb repressive complex 2 subunit; CXCR4, C-X-C motif chemokine receptor 4; WNT1, Wnt family member 1; CCND1, cyclin D1; PI3K, phosphatidylinositol 3-kinase; AKT, AKT serine/threonine kinase; NF-κB, Nuclear factor-Κb.
7.3 Epigenetic modification of gene expression
Polycomb repressive complex 2 (PRC2) is an extremely conserved protein complex capable of silencing gene expression by methylating lysine 27 on histone H3. Histone methyltransferase enhancer of zeste homolog 2 (EZH2) is a catalytic subunit of PRC2 [106]. LncRNA SNHG1 can act as an oncogenic molecule of CCA by binding to EZH2, followed by epigenetically suppressing the transcription of CDKN1A in the nucleus [49]. CDKN1A, as a target of the tumor suppressor p53, encodes a potent cyclin-dependent kinase inhibitor and induces the apoptosis of cancer cells [107]. The FBP1 gene encodes a rate-limiting gluconeogenic enzyme, the loss of which can accelerate cancer progression by enhancing aerobic glycolysis, and the antineoplastic role of FBP1 has been verified in multiple cancers including CCA [108]. Additionally, upregulated DANCR can promote CCA progression through the epigenetically transcriptional inactivation of FBP1 [52]. In addition, NEAT1 and PVT1 are also able to recruit EZH2 and increase H3K27me3 at the promoters of E-cadherin and ANGPTL4, respectively, in CCA [43, 60] (Figure 3A).
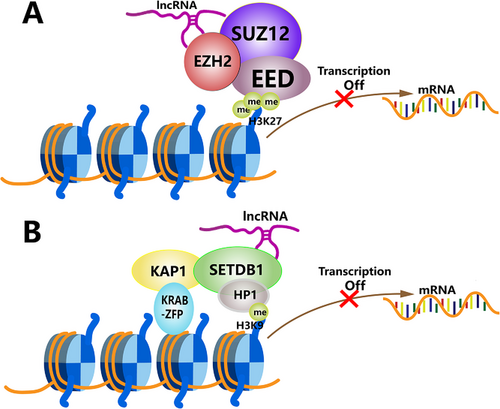
A study by Tsai et al [109] showed that lincRNA HOTAIR could serve as a scaffold for at least two distinct histone modification complexes. In cholangiocarcinoma, Xu et al [20] discovered that lncRNA SPRY4-IT1, as a ceRNA for miR-101-3p, could bind to miR-101-3p and release its inhibition of EZH2 mRNA, leading to increased EZH2 protein levels. Subsequently, SPRY4-IT1 recruited EZH2 and DNMT1 to the KLF2 promoter region, and recruited EZH2 and LSD1 to the LATS2 promoter region, and repressed their transcription. Subsequent experiments determined that the oncogenic function of SPRY4-IT1 was partly dependent on inhibiting KLF2 and LATS2 expression [20]. Therefore, a lncRNA may play a role in promoting cancer by mediating multiple ways of epigenetic modifications.
The SETDB1 protein belongs to the SET-domain protein methyltransferase family and is involved in the trimethylation of H3K9 and silencing gene expression [110]. Emerging evidence shows that the aberrant overexpression of SETDB1 is closely related to the development of cancer, such as melanoma, breast cancer, and mesothelioma [111-113]. In a study by Qin et al [64], lncRNA FENDRR expression levels were significantly decreased in CCA, and FENDRR was capable of enriching SETDB1 in CCA cells. The overexpression of FENDRR inhibited the expression of survivin, a well-studied oncogene, via SETDB1-mediated H3K9 methylation to suppress the proliferation, migration, and invasion of CCA cells (Figure 3B) [64].
Abnormally expressed lncRNAs in CCA have been proven to mediate multiple epigenetic modifications of genes by serving as scaffolds for histone modification complexes. With the silencing of target gene expression, CCA development is promoted. DNA methylation mediated by lncRNAs has been discovered in hepatocellular carcinoma and colorectal cancer [114, 115]. There are still other patterns of epigenetic modifications mediated by lncRNAs in CCA urging further exploration.
7.4 Synergism and antagonism with host genes
Previous studies have demonstrated that the function of lncRNAs is partly dependent on their genomic location, and lncRNAs have been proposed to play essential roles in modulating the expression of nearby loci [116]. LncRNA AFAP1-AS1 is derived from the antisense DNA strand in the AFAP1 gene locus [117]. A recent study showed that AFAP1-AS1 was significantly upregulated in CCA and could promote the growth and metastasis of CCA cells. However, the knockdown of AFAP1-AS1 increased AFAP1 protein and mRNA levels [118]. The AFAP1 protein monitors actin filament integrity and functions as an adaptor protein linking members of the Src family and other signaling proteins involved in actin filaments [119]. Therefore, we deduced that the metastatic effects of AFAP1-AS1 on CCA cells may be mediated by altered AFAP1 protein levels.
The lncRNA CPS1-IT1 and its host gene CPS1 were observed to be highly co-expressed in iCCA tissue and associated with a poor iCCA phenotype. The high expression of CPS1 was observed not only at the mRNA level, but also at the protein level. Furthermore, CPS1 and CPS1‑IT1 knockdown inhibited iCCA cell proliferation and accelerated cell apoptosis [120]. CPS1 protein is traditionally regarded as the first key enzyme in the urea cycle, which occurs only in the liver and can convert toxic ammonia into less toxic urea [121]. Due to the accumulation of ammonia, the translation of ODC mRNA, the polyamine biosynthetic rate-limiting enzyme, decreased significantly, thus inhibiting polyamine biosynthesis and cell proliferation [122]. Cancer progression is frequently accompanied by rearrangements of metabolic pathways. Perhaps to meet the abnormal metabolic demands of CCA cells, the urea cycle is likely to be accelerated in CCA.
The upregulation of lncRNA SOX2-OT can promote the progression of CCA by activating the PI3K/AKT signaling pathway. In addition, the positive regulatory effects of SOX2-OT on SOX2 have been verified in CCA cells [39]. Since the SOX2 gene is embedded in the intronic region of SOX2-OT, SOX2-OT can regulate the expression of SOX2 to affect the biological behaviors and stemness of various cancers, such as breast cancer, osteosarcoma, and esophageal squamous cell carcinoma [123-126]. In addition, previous studies have shown that the expression of SOX2 was associated with aggressive behavior and poor overall survival in iCCA [127]. However, the specific mechanism by which SOX2-OT activates the transcription of SOX2 remains unclear. Synergism and antagonism between lncRNAs and their host genes are still just macro conceptions, and how lncRNAs specifically regulate the expression of host genes and how they interact with each other remains unknown.
7.5 Cancer-related signaling pathways regulated by lncRNAs
7.5.1 PI3K/AKT signaling pathway
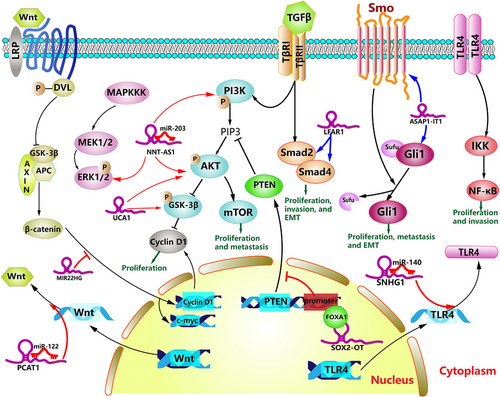
Dysregulation of lncRNA expression can promote the development of CCA by activating diverse oncogenic signaling pathways. The PI3K/AKT signaling pathway is aberrantly activated in various cancers, including cholangiocarcinoma, and loss of function of the tumor suppresser gene PTEN can stimulate the activation of this pathway [128]. FOXA1 is a pioneer transcription factor, and its mutation can promote the initiation and metastatic progression of prostate cancer [129]. According to the study of Wei et al [39], FOXA1 can directly bind to the PTEN promoter region and inhibit PTEN transcriptional activity in CCA cells. Furthermore, lncRNA SOX2-OT positively regulates the phosphorylation of PI3K and AKT in CCA cells partially by interacting with FOXA1 in the nucleus to inhibit PTEN transcription [39]. Similarly, lncRNA UCA1 knockdown results in significantly lower phosphorylation levels of AKT and GSK3β. This inhibition further downregulates the expression of CCND1 and suppresses the G1/S transition of CCA cells [83]. The phosphorylation levels of PI3K and AKT were also observed to be notably increased by both NNT-AS1 and MALAT1 overexpression in CCA [96, 130]. Multiple lncRNAs participate in the progress of CCA by regulating the phosphorylation of key proteins in PI3K/AKT signaling pathway.
7.5.2 MAPK signaling pathway
ERK, as a mitogen-activated protein kinase (MAPK) pathway, has been proven to regulate various cellular biologic behaviors such as proliferation, invasion, and migration. Menakongka et al. [131] found that the promotion of ERK1/2 played a positive role in CCA cell invasion. In addition, overexpression of NNT-AS1 could significantly increase the phosphorylation level of ERK1/2 [96]. Remarkably, vimentin, which is traditionally regarded as a key member of EMT, has been shown to be a novel downstream effector of AKT, and the ERK1/2/Slug/E-cadherin signaling pathway has been found to play an important role in promoting the invasiveness of gastric cancer [132, 133]. Therefore, it is reasonable to assume that there is a complex regulatory network between different oncogenic signaling pathways and EMT in tumor development.
7.5.3 Wnt/β-catenin signaling pathway
The Wnt/β-catenin signaling pathway involves the stabilization of β-catenin and the activation of downstream target genes such as c-myc and cyclin D1, which are all oncogenes in tumor progression. Emerging evidence demonstrates that abnormal activation of the Wnt/β-catenin signaling pathway plays a significant role in the initiation and development of CCA [134]. Hu et al [66] found that MIR22HG, a downregulated lncRNA in CCA, could negatively regulate the expression levels of Wnt/β-catenin signaling pathway-related proteins (β-catenin, cyclin D1, and c-myc), while activation of the Wnt/β-catenin signaling pathway partly abrogated the adverse impact of MIR22HG overexpression on CCA progression. The translocation of β-catenin is a pivotal step for activation of the Wnt/β-catenin pathway, and the nuclear translocation of β-catenin was also inhibited after the overexpression of MIR22HG [66].
An investigation by Zhang et al [135] revealed that the upregulation of lncRNA PCAT1 facilitated eCCA cell growth and inhibited apoptosis by targeting the Wnt/β-catenin signaling pathway. The expression of Wnt1, which is a member of the WNT family, was observed to be negatively regulated by miR-122 with its 3’- untranslated region binding to miR-122, and PCAT1 could relieve this negative regulatory effect of miR-122 on Wnt1 [135]. As an important signaling pathway in CCA development, Wnt/β-catenin signaling pathway can be regulated by lncRNAs in different cellular localizations.
7.5.4 Hedgehog signaling pathway
The Hedgehog (Hh) signaling pathway was first identified in the fruit fly. The aberrant activation of the Hh signaling pathway is caused by mutations in related genes or by the excessive expression of Hh signaling molecules, such as Patched (Ptch), Smoothened (Smo), and Glioma-associated oncogene 1 (Gli1), whose abnormal expression might contribute to the onset of multiple tumors [136, 137]. It has been verified that lncRNAs can activate their downstream signaling pathway, thus promoting the progression of skin cancer and colorectal cancer [138, 139]. LncRNA ASAP1-IT1 can activate the Hedgehog signaling pathway by positively modulating Smo and Gli1 in CCA cells to promote the development of CCA [51]. Unfortunately, the specific mechanism of action between ASAP1-IT1 and these downstream molecules was not well described in the current studies.
7.5.5 TGF-β/Smad signaling pathway
The TGF-β/Smad signaling pathway participates in a wide range of cellular processes, especially metabolic reprogramming of cancer-associated fibroblasts, and lnc-LFAR1 is involved in the progression of liver fibrosis by regulating the TGF-β/Smad signaling pathway [140-142]. Chen et al. found that lnc-LFAR1 was highly expressed in iCCA cells, and the overexpression of lnc-LFAR1 could promote iCCA cell proliferation and invasion via the upregulation of TGF-β1, Smad2, and Smad4 [46]. Accumulating evidence suggests that the downstream biological effects of the TGF-β pathway depended not only on distinct Smad proteins but also on the interaction with numerous noncanonical signaling factors, including PI3K/Akt, ERK, and JNK/p38 [143]. For instance, MALAT1 knockdown significantly suppressed the protein level of noncanonical TGF-β signaling proteins (PI3Kp85α and p-Akt) in human osteosarcoma cells [144]. Many noncanonical signaling factors are involved in the TGF-β signaling pathway. Therefore, future research should pay more attention to the role of lncRNAs in the cross-talk between different signaling pathways in CCA.
7.5.6 NF-κB signaling pathway
Nuclear factor-κB (NF-κB) is an important and evolutionarily conserved transcription factor involved in coordinating immune and inflammatory responses [145]. In recent years, numerous studies [146] have confirmed that the NF-κB pathway is constitutively activated in various cancers, which leads to the occurrence and progression of cancer via the upregulation of multiple antiapoptotic and oncogenic genes. The Toll-like receptor (TLR) superfamily is one of the most common NF-κB-activating signals [146]. Overexpression of lncRNA SNHG1 in CCA accelerated the expression of TLR4 and activated the NF-κB pathway, thus regulating the growth and tumorigenesis of CCA [97]. Lu et al [147] confirmed that the downregulation of lncRNA MEG3 in CCA cells induced the overexpression of miR-361-5p, resulting in the inhibition of TRAF3 expression. The miR-361-5p inhibitor significantly repressed the expression of the p-p65 protein, a key member of the NF-κB family, and this effect was eliminated by TRAF3 knockout. Under normal circumstances, NF-κB-inducing kinase (NIK) is continuously degraded by a TRAF3-dependent E3 ubiquitin ligase. As a result of TRAF3 degradation, NIK becomes stabilized, leading to the activation of the noncanonical NF-κB pathway [148]. Interestingly, the downregulated lncRNA-NEF induced high expression of RUNX1 in iCCA [67]. However, RUNX1, a member of the Runt-related transcription factor 1 family, has been proven to serve as an attenuator of the NF-κB signaling pathway in gastric cancer and myeloid tumors [149, 150], indicating that the NF-κB signaling pathway may play a dual role in cancer, including CCA.
The activation of diverse cancer-related signaling pathways plays a decisive role in the progression of CCA. However, the involvement of lncRNAs in the Hedgehog pathway has not been well described in CCA. Interestingly, in other kinds of cancers, activation of the Hedgehog pathway mediated by lncRNAs leads to modulation of cancer stem cell properties and glucose metabolism reprogramming [151, 152]. In Figure 4, we illustrated these pathways regulated by lncRNAs in CCA.
8 CLINICAL VALUE OF lncRNAs IN CCA
8.1 Correlation between lncRNAs and clinicopathological features of CCA patients
Several lncRNAs were demonstrated to be statistically correlated with the clinicopathological features of CCA patients, such as TNM stage, tumor size, postoperative recurrence, lymph node invasion, and tumor differentiation. For example, patients with overexpression of TP73-AS1 had larger tumor size (P = 0.008) and more advanced TNM stage (P = 0.026) compared with the low TP73-AS1 expression group patients [22]. It is a common view that tumor size >5 cm is associated with microscopic vascular invasion and higher tumor grade, which implies a worse prognosis [153]. It is worth noting that according to the 8th edition of the American Joint Committee on Cancer (AJCC) staging manual, the periductal invasion was removed from the T4 category due to a lack of recent data on the prognostic effect, which indicates that the TNM stage of CCA patients’ needs to be carefully classified in future studies [154]. Interestingly, lnc-PKD2-2-3 levels were significantly associated with abnormal CEA levels (P = 0.024) and poor differentiation (P = 0.002), and the upregulation of lncRNA CPS1-IT1 was associated with higher levels of CA19-9 (P = 0.044) [87, 120]. To provide a more comprehensive understanding, we summarized the dysregulated lncRNAs and their correlation with the clinicopathological features of CCA in Table 3. None of the lncRNAs were significantly associated with patient age or gender.
| LncRNA | Expression | Tumor stage (T1-T2/T3-T4) | TNM stage (I-II/III-IV) | Postoperative Recurrence (present/absent) | Lymph Node Invasion (present/absent) | Tumor size (cm) | Differentiation (well/poor) | Progression-free survival (PFS) | Overall survival (OS) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| AFAP1-AS1 | ↑ | - | √ | - | - | √(5) | - | - | √ | [118] |
| ASAP1-IT1 | ↑ | √ | √ | √ | √ | - | × | - | √ | [51] |
| CCAT2 | ↑ | √ | √ | √ | √ | √(3) | × | √ | √ | [56] |
| CCAT2 | ↑ | √ | √ | √ | - | ×(5) | √ | √ | √ | [70] |
| CPS1-IT1 | ↑ | - | - | - | √ | × | - | √ | × | [120] |
| CRNDE | ↑ | √ | √ | - | × | √(5) | √ | √ | √ | [69] |
| H19 | ↑ | - | √ | √ | × | √(3) | × | - | √ | [179] |
| HOTAIR | ↑ | - | √ | √ | × | √(3) | × | - | √ | [57] |
| KCNQ1OT1 | ↑ | √ | √ | √ | √ | - | × | - | √ | [48] |
| LINC00261 | ↑ | - | √ | √ | √ | √(3) | × | - | √ | [23] |
| LINC01296 | ↑ | - | √ | - | √ | √(3) | - | - | √ | [58] |
| LINC01410 | ↑ | - | - | - | - | - | - | - | √ | [50] |
| LINC01714 | ↓ | - | - | - | - | - | - | - | √ | [63] |
| LOXL1-AS1 | ↑ | - | √ | - | √ | ×(3) | × | - | √ | [59] |
| MALAT1 | ↑ | √ | - | - | × | √(3) | - | - | √ | [130] |
| MEG3 | ↓ | - | √ | - | √ | - | × | - | √ | [35] |
| MIR22HG | ↓ | - | √ | - | √ | √(5) | - | - | √ | [66] |
| NNT-AS1 | ↑ | - | √ | √ | - | √(3) | × | √ | √ | [100] |
| NNT-AS1 | ↑ | - | √ | - | √ | ×(5) | - | √ | √ | [54] |
| PKD2-2-3 | ↑ | × | √ | - | - | ×(5) | √ | - | √ | [87] |
| SNHG3 | ↑ | - | √ | - | √ | ×(3) | × | - | √ | [68] |
| SNHG6 | ↑ | - | - | - | - | - | - | - | √ | [76] |
| SOX2-OT | ↑ | √ | √ | √ | √ | - | × | - | √ | [39] |
| SPRY4-IT1 | ↑ | √ | √ | - | × | - | × | √ | √ | [20] |
| TP73-AS1 | ↑ | - | √ | - | × | √(3) | × | - | - | [22] |
| TUG1 | ↑ | √ | √ | √ | √ | √(3) | × | √ | √ | [78] |
| UCA1 | ↑ | √ | √ | √ | √ | - | × | - | √ | [44] |
8.2 Diagnostic and prognostic value of lncRNAs
Compared with mRNAs and microRNAs, lncRNAs are expressed in a more cell-type- and tissue-specific manner, and therefore, lncRNAs may have more advantages as biomarkers for the diagnosis and prognosis of CCA [1]. As mentioned above, many studies have demonstrated that upregulated lncRNAs are related to aggressive tumor phenotypes and unfavorable prognosis, suggesting that lncRNAs could function as potential molecular biomarkers of the prognosis of CCA patients [155]. For instance, higher expression levels of SPRY4-IT1 indicated worse progression-free survival (PFS) and overall survival (OS) after radical surgery in CCA patients [20]. Cox regression analyses confirmed that SPRY4-IT1 could be regarded as an independent predictor of adverse PFS (P = 0.018) and OS (P = 0.027) in CCA patients [20]. Other lncRNAs associated with PFS and OS of CCA patients are summarized in Table 3.
In the iCCA-related ceRNA regulatory network constructed by Xu et al [103], ROC analysis based on the NCBI GEO database and the TCGA database revealed that lncRNA RP11-328K4.1, miRNA hsa-miR-27a-3p, and mRNA PROS1 could distinguish between iCCA tumor tissues and matched adjacent nontumor tissues, and particularly, hsa-miR-27a-3p might have significant diagnostic value in identifying iCCA of different clinical stages. Currently, serum levels of CA19-9 is the major tumor marker for iCCA diagnosis, but the sensitivity and specificity of CA19-9 are only 62% and 63%, respectively [13]. Ma et al [120] found that with increasing expression of lncRNA CPS1-IT1, the CA19-9 positive rate was greater (P = 0.044), indicating that CPS1‑IT1 may serve as a potential biomarker for the diagnosis of iCCA. Shi et al [156] examined the expression of the 12 candidate lncRNAs in perihilar cholangiocarcinoma patients and healthy controls, and found that the combination of PCAT1, MALAT1, and CPS1-IT1 presented a high sensitivity (85.5%) and specificity (93.2%) with an area under the ROC curve value (AUC) of 0.893. Therefore, plasma levels of these three lncRNAs might function as a signature for predicting perihilar cholangiocarcinoma [156].
8.3 Adjuvant effect of lncRNAs on chemotherapy
The combination of gemcitabine and cisplatin is the standard cytotoxic treatment for advanced/metastatic cholangiocarcinoma, but unfortunately, it confers a median survival of only approximately 1 year [8]. Shen et al. [63] found that LINC01714 knockdown dramatically increased the half-maximal inhibitory concentrations (IC50s) of gemcitabine in gemcitabine-treated CCA cells, while the overexpression of LINC01714 led to the opposite result. Subsequent experiments revealed that LINC01714 enhanced the cytotoxic effect of gemcitabine on CCA cells by modulating phosphorylated FOXO3-Ser318. This observation suggests that LINC01714 may act as a candidate for combinatorial chemotherapy with gemcitabine for CCA patients (Figure 5A).
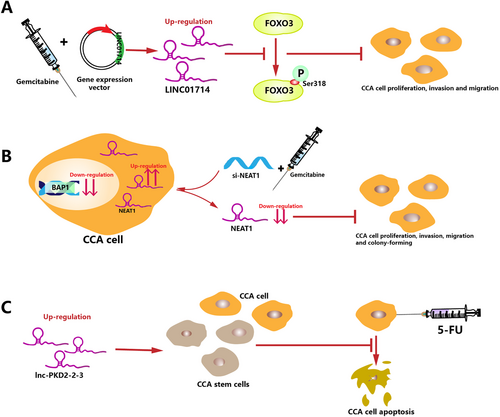
Mutations in the chromatin modulator BRCA-1 associated protein-1 (BAP1) have been identified as the most frequent genetic alterations in CCA, occurring in 22%-24% of cases [157]. Parasramka et al [158] found that BAP1 expression could modulate CCA cell sensitivity to gemcitabine, or more specifically, sensitivity to gemcitabine was increased in low BAP1-expressing CCA cells than in high BAP1-expressing CCA cells. Moreover, lncRNA NEAT1 was identified to be enriched after BAP1 knockdown, and researchers further confirmed that NEAT1 could serve as a functional downstream target of BAP1 involved in drug responses [158] (Figure 5B). Previous preclinical data indicated that DZNep, an EZH2 inhibitor, could induce apoptosis and G1 phase cell cycle arrest in CCA cells, and compared with gemcitabine alone, the combination of DZNep and gemcitabine enhanced the induced apoptosis and G1 arrest [159]. However, in the study by Parasramka et al [158], the sensitivity to the combination of EZH2 inhibition and gemcitabine was reduced in low-BAP1 CCA cells compared with high-BAP1 CCA cells. Since the recruitment effect of NEAT1 on EZH2 has been confirmed in CCA, low BAP1-expressing cells may have a higher expression of NEAT-1 and EZH2, resulting in lower sensitivity to EZH2 inhibition [60]. Therefore, the expression levels of BAP1, NEAT1, and EZH2 should be evaluated in future studies to institute an accurate clinical treatment plan.
5-Fluorouracil (5-FU) is a first-line chemotherapeutic drug for CCA but its efficacy has been curbed with the development of drug resistance [160]. However, emerging evidence suggests that the proportion of cells with high expression of cancer stem cell (CSC) markers among the surviving cells is significantly increased in response to 5-Fu, resulting in drug resistance and disease relapse. This phenomenon has been discovered in various cancers, including esophageal adenocarcinoma [161], hepatocellular carcinoma [162], gastric cancer [163], and non-small cell lung cancer [164]. Lnc-PKD2-2-3 is one of the upregulated lncRNAs identified in CCA and is associated with an aggressive phenotype and poor prognosis. Qiu et al [87] proposed lnc-PKD2-2-3 as a novel CSC marker in CCA. With the increase in lnc-PKD2-2-3 levels in CCA cells, the expression of the apoptotic marker caspase-3, as well as the apoptosis rate of cells, were decreased after 5-FU treatment. Consequently, it may be feasible to utilize lnc-PKD2-2-3 as a target to eliminate 5-FU resistance in CCA patients (Figure 5C).
9 FUTURE PERSPECTIVES
Only a few studies have discovered that there were differences in the epidemiological features between iCCA and eCCA, especially the risk factors. Hepatitis C virus (HCV) infection was reported to be positively correlated with an increased risk of iCCA but not with eCCA. Additionally, smoking, alcohol consumption, and non-alcoholic fatty liver disease have a stronger correlation with iCCA, while most bile duct diseases are more strongly correlated with eCCA [165]. In the study by Xu et al(20), lncRNA SPRY4-IT1 was found to be highly expressed in iCCA cell lines but not in pCCA or dCCA cell lines, suggesting that different anatomic types of cholangiocarcinoma may have different patterns of gene mutations. Breast cancer 1 (BRCA1) is a classical tumor suppressor gene. In a study of 935 patients with cholangiocarcinoma, the mutation frequency of BRCA1 in eCCA samples was five times that in iCCA samples [166]. LncRNA GUARDIN is capable of sustaining breast cancer 1 (BRCA1) stability by acting as an RNA scaffold to facilitate the heterodimerization of BRCA1 and BRCA1-associated RING domain protein 1 [167]. Therefore, exploring whether there is a specific change in lncRNA GUARDIN function or level in eCCA may be conducive to the reclassification of cholangiocarcinoma from the perspective of gene patterns. Further investigation into the role of lncRNAs in CCA should first clarify the classification of CCA samples and then focus on specific mechanisms of the regulation of mRNA and protein levels.
Currently, the functions of numerous lncRNAs in CCA remain unknown. For instance, miR-34a is significantly decreased in human CCA tissues compared with adjacent non-tumor tissues, and participates in downstream cascades of TGFβ1-mediated CCA development [168]. In other cancer types, miR-34a has been shown to interact with a variety of lncRNAs [169, 170]. However, whether lncRNAs are involved in the regulation of miR-34a in CCA remains to be further studied.
Additionally, the structures of lncRNAs have received little attention, and structure-function relationships remain unclear; therefore, limitations still exist in studies on the mechanisms of lncRNAs in CCA. Given that current studies on lncRNA mechanisms converge on establishing intermolecular interactions with other molecules, such as proteins, DNA, and other RNAs, it is essential to elucidate the lncRNA-protein and lncRNA-DNA interactions they engage in. Recent studies [171, 172] have reported that lncRNAs function by forming RNA-DNA triple helixes with DNAs. In tongue squamous cell carcinoma, lncRNA CISAL can be tethered to the gene promoter through RNA-DNA triple helix formation, spatially sequestering transcription factors away from DNA-binding sites and restraining transcription of tumor suppressor genes [171]. LncRNA Fendrr can form RNA-DNA triple helixes in the promoter region of developmental genes and then serve as an anchor to recruit the histone-modifying protein PRC2 [172]. At present, there are only a few studies on the role of RNA-DNA triple helixes in carcinogenesis, and it is still necessary to reinterpret the epigenetic modifications of genes mediated by lncRNAs that have already been elucidated in CCA.
An example of extracellular vesicles (EVs), exosomes are self-contained vesicles with a lipid bilayer released by parent cells. These vesicles can mediate cellular communications by transmitting active molecules, including lncRNAs, and then induce epigenetic regulation, cellular reprogramming, and genomic instability in recipient cells, ultimately enabling the generation of cancer-initiating cell phenotypes and resistance to chemotherapies [173]. Hypoxic bladder cancer cells can facilitate tumor development by secreting oncogenic lncRNA-UCA1-enriched exosomes, and exosomal lncRNA-UCA1 in human serum has been proven to possess potential as a diagnostic biomarker for bladder cancer [174]. Kitdumrongthum et al. [175] identified distinct miRNA signatures in exosomes released from CCA cells compared to normal human cholangiocyte cells, and some of these signatures may have the potential to be novel biomarkers for CCA. Unfortunately, no studies on serum or tissue exosomal lncRNAs in patients with CCA have been conducted so far. Due to the deep anatomical location and lack of sensitive biomarkers in the early stage, most CCA patients are diagnosed at a late stage. Therefore, subsequent studies should probably focus on lncRNAs detectable in serum or bile secretions.
In view of the important role of lncRNAs in CCA, modulation of lncRNA expression has great potential for gene-based therapy. For those highly expressed lncRNAs, compared with those of cells transfected with lncRNA mimic, the malignant behaviors of CCA cells were significantly attenuated after transfection with lncRNA inhibitor (shRNAs/siRNAs). ShRNAs/siRNAs are collectively referred to as RNA interference molecules. RNA interference is one of the most powerful techniques for gene silencing, where RNA fragmented into short RNAs suppress the expression of homologous genes by complementary RNA cleavage [176]. Kim et al. [177] combined standard temozolomide treatment with targeted nanocomplexes carrying siRNA against lncRNA MALAT1 to treat animal models of glioblastoma (GBM), and then significantly weakened the growth, motility, and stemness of GBM cells. The discovery of extracellular vesicles (EVs), especially exosomes, as natural carriers of functional small RNAs and proteins has raised great interest in the drug delivery field, as it may be possible to utilize these vesicles for therapeutic delivery of miRNAs, lncRNAs, and their inhibitors. With further development of engineered exosome mimetics and glycosylphosphatidylinositol (GPI)-anchored nanobodies on EVs, this kind of therapy delivery tool will overcome targeting difficulties and be more acceptable pharmaceutically [178].
10 CONCLUSION
Cholangiocarcinoma (CCA) is a heterogeneous malignancy arising from complicated interactions between host genetic background and various risk factors. The incidence rate of CCA is rising globally. However, due to the lack of specific symptoms and sensitive diagnostic indicators, current modalities for the diagnosis of CCA are insufficient. With the widespread application of advanced epigenomic technologies and deep RNA-sequencing, a growing number of long noncoding RNAs (lncRNAs) are being identified at a phenomenal rate, and their aberrant expression is closely related to the occurrence and development of multiple cancers, including CCA. In this review, we summarized the functional studies and bioinformatics analyses on the role of lncRNAs in the development of cholangiocarcinoma. The malignant proliferation of cholangiocytes is an unfavorable outcome of cholestatic liver diseases, and lncRNAs could be playing an important role in this process. Dysregulation of lncRNA expression was found to be significantly correlated with the clinicopathological features of CCA patients and the biological characteristics of CCA cells. The abnormal activation of diverse cancer-related signaling pathways plays a decisive role in the progression of CCA, and lncRNAs have been proved to be the key molecules to activate these pathways. Furthermore, lncRNAs can mediate epigenetic modification at the transcriptional level or function as ceRNAs to compete with mRNAs for sponging miRNAs in CCA. Interestingly, some lncRNAs can facilitate CCA progression by regulating the transcription and translation of their host genes or directly binding with functional proteins. Due to their tissue-specific expression in a regulated manner, lncRNAs have considerable diagnostic and prognostic value in CCA patients. In addition, some preliminary drug sensitivity tests have indicated that lncRNAs might be promising novel therapeutic targets of CCA.
DECLARATION
AUTHORS’ CONTRIBUTIONS
LM and QJ conceived and supervised the review. FW and QPL collected relevant literature and proposed scientific inputs. YY and XTD drafted the manuscript. LM revised the manuscript. All authors have read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICTS OF INTEREST
None.
FUNDING
This work was supported by the National Key Research and Development Program “precision medicine research” (No. 2017YFC0908304), the Project of Jiangsu Provincial Key medical talents in Jiangsu Province (ZDRCA2016034), the National Natural Science Foundation of China (81801105), and the Natural Science Foundation of Jiangsu Province (BK20181092).
ACKNOWLEDGMENTS
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




