Nucleic acid delivery by ionizable nanocarriers for brain disease treatment
[Correction added on 27 June 2023, after first online publication: The copyright line was changed.]
Abstract
The successful application of messenger RNA vaccines in the market has demonstrated the potential of gene therapy in treating various diseases, including infectious diseases, autoimmune disorders, brain diseases, and other cancers. However, gene therapy faces great challenges in treating brain diseases such as brain tumors, infections, and strokes because the limitations of the blood-brain barrier make it difficult for nucleic acid drugs to be delivered safely and effectively into the brain. Therefore, there is a high demand for carriers delivering nucleic acid drugs to the brain. Ionizable nanocarriers (INs) have great advantages in gene therapy due to their pH-responsive properties, which facilitate the safe and efficient delivery of targets, responsive release in the disease microenvironment, and the protection of nucleic acids from degradation. To better understand INs and their potential as therapeutic vectors for brain diseases, the present review describes their biological properties, recent progress in the field, and promising applications. In particular, the related prospects and challenges are discussed to promote the further development of INs.
Key points
What is already known about this topic?
-
Ionizable nanocarriers (INs) have great advantages in brain-targeted gene therapy due to their pH responsiveness, which are conducive to the safe and efficient delivery across the blood-brain barrier, responsive release in the microenvironment of brain diseases, and protection of nucleic acids from degradation, and have good clinical application potential.
What does this study add?
-
To better understand INs and their potential as carriers in treating brain diseases, the present review describes the biological characteristics and categories of INs, the nucleic acids delivered (siRNA, mRNA, pDNA, and ASOs), and brain diseases, such as glioblastoma and stroke. In particular, the microenvironment of brain diseases, the delivery strategies of nucleic acids by INs, and the clinical transformation are discussed.
1 INTRODUCTION
Brain diseases are a major global health burden including a wide range of conditions, such as acute disseminated encephalomyelitis (infections), cerebral atrophy (trauma), epilepsy (seizures), gliomas (tumors and masses), stroke (vascular conditions), multiple sclerosis (autoimmune conditions), and Alzheimer's disease (AD), neurodegenerative conditions.1
Despite recent advances in drug development, brain disease therapies still have a low success rate due to less understanding of the major barrier of the brain tissue, which make it challenging for molecules to reach the brain.
Nucleic acid drugs hold great promise in treating brain diseases through the correction of abnormal genes. Compared with small molecules and biological drugs, nucleic acid drugs offer strong specificity, rich gene targets, and long-lasting efficacy. They can encode related proteins of brain diseases, silence-specific gene expression, and activate gene transcription, and so on. Unlike small molecules and protein drugs, nucleic acids must function in the cell or even in the nucleus. However, nucleic acids are easily degraded by nucleases in the body and have difficulty in penetrating the blood-brain barrier (BBB) because of its negative charge. The brain microvascular endothelial cells (BMECs) present a significant obstacle to the delivery of solutes and macromolecules (such as anticancer drugs) to brain tissue.2 Furthermore, the transcellular transport of drugs is limited by efflux transporters (such as P-gp) expressed in BMECs, which removes various substrates of the cell for recycling, thus hindering the penetration of most conventional drugs and nearly all biological and nanopharmaceutical preparations into the brain.3 Therefore, there are still several barriers to treat brain diseases by nucleic acid drugs.
Ionizable lipid nanoparticles (ILNPs) have been used for messenger RNA (mRNA) delivery in humans and represent a promising approach for the clinical transformation of nanocarriers. The use of mRNA-ILNPs for COVID-19 (Corona Virus Disease 2019)4 vaccines has brought more attention to nucleic acid drugs, and their use has gradually expanded from the initial treatment of COVID-19 to that of common diseases. However, the treatment of brain diseases is more challenging than that of other diseases because of the existence of barriers to the safe and efficient delivery of nucleic acids across the BBB and into the brain.2
As evidenced by the successful approval of mRNA-ILNPs, the latest successful cases are two FDA-approved mRNA-LNP anti-COVID-19 vaccines developed by BioNTech and Moderna, respectively. These two vaccines are developed and marketed at an unparalleled speed and have significant effects in the prevention of epidemic viruses. Delivering nucleic acids to target cells by clinically translatable ionizable nanocarriers (INs) provides opportunities to tackle a series of health problems. With the rise of gene therapy, a new era of disease treatment has begun with a focus on the selective delivery of nucleic acids to specific organs or tissues2, 3 using INs. These INs can enhance the delivery of nucleic acids like DNA, mRNA, and small interfering RNA (siRNA)5-7 through a unique mechanism that involves a change in pH values. In a neutral environment, INs are in a nucleic acid-lipid complex form, while in an acidic environment, the double-layer structure of the INs is disrupted, promoting the release of nucleic acid from endosomes. Optimizing the physicochemical properties of nanocarriers is an effective strategy for brain-targeted RNA delivery and gene silencing.
Recently, great progress has been made in the treatment of brain diseases by encapsulating nucleic acids in INs. Researchers at Tufts University have developed a neurotransmitter-derived lipid (NT lipid) that utilizes neurotransmitters to aid lipid nanoparticles (LNPs) carrying antisense oligonucleotides (ASOs) in crossing the BBB and reaching the mouse brain. This innovation overcomes many limitations currently inherent to delivering therapeutic drugs to the brain and opens up possibilities for other therapies that cannot enter the brain.5 Min et al. loaded ASOs into glucose-modified ionizable polymer nanocarriers to generate ASO@IPLN (ASO—ionizable polymer-lipid nanoparticles). Subsequent experiments showed that these INs can target the brain to transport nucleic acids through the BBB, significantly reducing the mRNA translation of inflammatory genes in regions, including the cerebral cortex and hippocampus, for the treatment of CNS diseases.8 Similarly, an LNP containing ionizable aminolipids has recently been developed as a carrier for encapsulating siRNA, improving the siRNA delivery efficiency in mouse brain endothelial cells.9 An ionizable hybrid lipid self-assembled nanoparticle was also developed for microRNA (miRNA) delivery to the brain for treating glioblastoma.10 For the brain-targeting delivery of plasmid DNA (pDNA), different ionizable lipid/pDNA ratios were prepared by adding pDNA to ILNPs. The ILNP showed good transfection efficiency after administration in the rat's cerebral cortex.11
Thanks to intensive research efforts, there are a growing number of INs for the nucleic acid delivery to treat brain diseases recently. In the present review, we introduce the delivery strategies across BBB, as well as the different types of INs and their applications in the treatment of brain diseases, and identify common issues in the delivery of nucleic acids via INs (shown in Figure 1). Overall, progress in the use of INs for delivering nucleic acids for brain disease therapy has been promising and is continuing to advance.
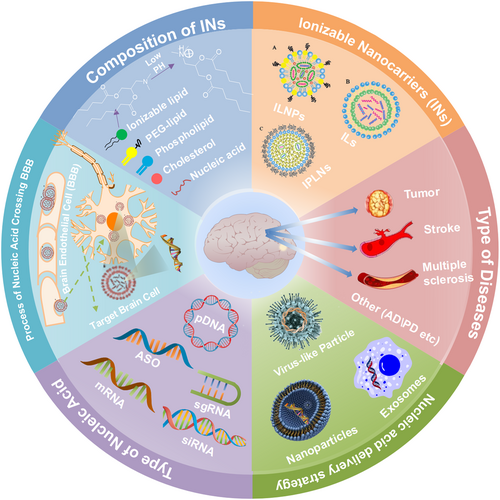
The delivery of nucleic acids by INs for brain diseases. ASO, antisense oligonucleotide; IL, ionizable liposome; ILNP, ionizable lipid nanoparticle; INs, ionizable nanocarriers; mRNA, messenger RNA; PEG, polyethylene glycol; siRNA, small interfering RNA.
2 BLOOD-BRAIN BARRIER (BBB) AND DELIVERY STRATEGIES
The treatment of brain diseases like tumors, infections, strokes, and neurodegenerative diseases is limited by the difficulty of safely delivering small drugs, peptides, and proteins to the brain across the BBB. Nanocarriers show great advantages to overcome this barrier and improve the delivery of drugs to the brain.2, 3
2.1 BBB
The BBB is a crucial component of the human body as it helps regulate substances that enter the brain and protect the CNS. This barrier is made up of a layer of endothelial cells within the cerebral blood vessels that allow only highly selective molecules from the bloodstream to penetrate and enter the fluid surrounding neurons and other cells in the brain. However, this also presents a challenge for medical professionals attempting to transport therapeutic molecules safely and effectively into the brain.
The neurovascular unit, which is made up of neurons, glial cells, and vascular cells, is essential for preserving homeostasis and normal brain functions. The brain vascular endothelial cells form the basis of the BBB and are responsible for its selective permeability through tight junctions.6 This selectivity helps maintain the regulated microenvironment of the CNS but also makes it difficult for potential neurological drugs to reach the brain.
Disorders of brain endothelial cells are believed to be closely related to the pathophysiology of CNS diseases, making the delivery of nucleic acids (i.e., RNA or DNA) through the BBB to brain endothelial cells a potential strategy for treating brain diseases.
2.2 Delivery strategies across the BBB
The treatment of neurodegenerative diseases as well as brain tumors, infections, and strokes is limited by the challenge of safely transporting small-molecule drugs, peptides, and proteins to the brain. The BBB poses a major obstacle to the CNS drug delivery due to its ability to restrict the entry of neurotherapeutics into the brain.3, 7 Direct injection and barrier disruption approaches carry risks, including infection, tissue damage, and neurotoxicity. To overcome this obstacle, various strategies have been developed to facilitate drug delivery to the brain. Brain-targeted nanocarriers for nucleic acid delivery, such as exosomes, virus-like particles (VLPs), and lipid/polymer-based nanoparticles, are promising as non-viral vectors.2, 6 These systems can protect the nucleic acids from enzymatic digestion and promote the efficient crossing of the BBB and the uptake into brain cells. They have the advantages of low toxicity, low immune response, and controllable chemical structure. Although these delivery strategies support clinical applications by promoting drug delivery efficiency and decreasing drug toxicity, there are many barriers in the related basic research, production control, and clinical trials, resulting in extremely low clinical transformation rates. For example, the delivery of nucleic acids by exosomes needs to enter the cytoplasm of brain cells to function. To achieve this function, it will face technical challenges, such as low nucleic acid load, poor distribution of targeted brain tissue, low delivery efficiency, and high heterogeneity of exosome production. Efforts should be made to ensure the low immunogenicity and inflammation, high transduction efficiency, and easy mass production of gene delivery systems.
2.2.1 Exosomes
Exosomes are small secretory extracellular vesicles and useful for the brain-targeted nucleic acid delivery.7 Like the cell membrane, exosomes have a lipid bilayer structure that can effectively load hydrophobic and hydrophilic drugs and is especially suitable for the loading of nucleic acids.12 Cukovic et al. designed miRNA-encapsulated exosomes for the treatment of drug-resistant epilepsy and identified the differences that 12 miRNAs (including miR-142-3p, miR-223-3p, and miR-21-5p) significantly increased in epileptogenic tubers.13 Although exosomes can be further applied as nucleic acid carriers in the field of brain disease treatment, they have associated disadvantages, such as difficulty in loading large nucleic acids, difficulty in selecting suitable exosome mother cells, and low brain targeting efficiency. In addition, the clinical transformation of exosomes is difficult; thus, few products have officially entered the market.
2.2.2 Virus-like particles (VLPs) for nucleic acid delivery
A VLP is a viral protein assembly that can infect cells but lacks viral genetic material. This virus-derived vector can use viral characteristics to achieve the effective intracellular delivery of substances including drug molecules, such as mRNA, protein, or ribonucleoprotein. VLPs have the functions of encapsulating drugs and endosomal escape. Surface particles are designed to target different cell types. A non-enzymatic VLPs assembly was introduced by Breyne et al., which successfully delivers the pDNA-scBVs (a plasmid DNA) and supercharged proteins into the mouse brain confirmed by signals in the striatum brain region.14 pDNA-scBVs increased the targeting affinity of brain tissue and reduced the toxicity and immunogenicity of viral vectors. Although the use of VLPs to deliver nucleic acids to cells has been shown to be feasible, they are easily blocked by capillary endothelial cells due to the cells' negatively charged cell membranes, resulting in the entry of insufficient or even no doses of nucleic acids into the brain parenchyma. The retention of negatively charged sc proteins (silk collagen proteins) in cationic lipids can improve the translocation of sc proteins to the cytoplasm and increase the resistance of the loaded nucleic acids to nuclease degradation.15 Large-scale clinical production of VLPs is challenging.
2.2.3 Lipid- and polymer-based nanoparticles
Various nanocarriers have been investigated to overcome the BBB6, 7 for the nucleic acid delivery to the brain, such as lipid-based nanoparticles (ILNPs and ionizable liposomes [ILs]), polymeric nanoparticles (PNPs), lipid/polymer hybrid nanoparticles (ionizable polymer-lipid nanoparticles [IPLNs]), and conjugated peptides.6 Lipid-based nanoparticles are the most widely used non-viral vectors for nucleic acid delivery due to their stable nanostructure in physiological fluids and their fusion with negatively charged molecules.3 Among them, LNPs include cationic LNPs and ILNPs. Ionizable lipids have pH responsiveness and become positively charged in acidic environments. The chemical properties of ionizable lipids are relatively mild, while cationic lipids are permanently positively charged and have strong cytotoxicity. Since the application of ILNPs in brain diseases is still in its infancy, to reflect the advantages of ILNPs, other cationic LNPs were reviewed as a comparison. In particular, emerging INs further improved the delivery efficiency and safety of nucleic acids with the development of ionizable nanomaterials, indicating a more promising approach compared with cationic nanoparticles for treating brain diseases. The use of INs is a reasonable strategy for the targeted nucleic acid delivery to brain cells. For example, in recent reports, replacing unmodified cholesterol with ionizable oxidized cholesterol in the cKK-E12 nanoparticle formulation could increase the delivery efficiency of mRNA to brain endothelial cells five-fold. It also reduced toxicity and immunogenicity and prolonged systemic circulation time, which is conducive to clinical transformation.16
Over the years, the use of a variety of in vivo gene delivery technologies has been attempted to improve nucleic acid delivery. However, some of these tools still have associated problems of toxicity, immunogenicity, quality control, high cost, and inability to pass through the BBB. Among these vectors, INs, especially ILNPs, hold the most promise for clinical transformation, which has also been confirmed by COVID-19 mRNA vaccines. Therefore, it is necessary to explore INs and their applications in treating brain diseases in depth.
3 IONIZABLE NANOCARRIERS (INs)
As an advanced delivery platform, INs include ILNPs, ILs, and IPLNs in which the ionizable nanomaterials are critical for the delivery of polyanionic nucleic acids by the pH-responsive interaction. The advantages of INs are expected to promote the gene therapy with good biocompatibility. For nucleic acids, these INs can provide a stable loading rate, prolong the pharmacokinetics, improve the penetration into the BBB, thereby targeting to brain tissue efficiently.17 Therefore, IN-based nucleic acid delivery systems improve the low treatment index compared with traditional dosage forms and are a promising formulation choice.
3.1 Ionizable lipid nanoparticles (ILNPs)
As the most widely used INs, ILNPs are composed of ionizable lipids and auxiliary components, such as cholesterol, phospholipids, and polyethylene glycol (PEG). The key component of INs is pH-responsive ionizable lipids for the delivery of nucleic acids to the brain. The selected ionizable lipid typically contains a hydrophilic head group, a hydrophobic lipid chain, and a connecting part. In acidic environments, positively charged ionizable lipids can be connected to nucleic acids, making them more stable and reducing physiologically neutral side effects.18 Cholesterol plays a critical role to regulate the interaction between phospholipids and proteins, promoting membrane fusion and enhancing the stability of ILNPs.19 Amphiphilic PEG-lipids reduce the aggregation, avoid the endocytosis by immune cells, and improve the stability of ILNPs.20 The double-layer structure of ILNPs strengthens and protects phospholipids, further enhancing their stability.21
Kratzer et al. designed a protamine-oligonucleotide nanoparticle coated with apolipoprotein A-I, which increased nucleic acid uptake and endocytosis without adverse effects on brain capillary endothelial cells.6 In order to deliver nucleic acids to the brain tissue across the BBB, Ray et al. designed ILNPs using DLin-MC3-DMA as an ionizable lipid, which showed low immunogenicity, low toxicity, and the ability to cross the BBB for potential drug delivery to brain tissue.22
3.2 Ionizable liposomes (ILs)
ILs are excellent INs that are not charged in the blood circulation but can be protonated in endosomes and lysosomes with a lower pH environment. These ILs interact with apolipoproteins and are transferred to brain tissue. The main ILs include asymmetric liposome particles (ALP) and O′,O-(3-(dimethylamino)propane-1,2-diyl)16-bis(2-(2methyl-5-nitro-1H-imidazol-1-yl)ethyl)di(hexadecanedioate) liposomes (MLP). Liu et al. reported that ILs deliver siRNA to selectively treat glioblastoma (GBM) in low pH and hypoxic environments. The authors synthesized ILs-MLP, which contains a malate dehydrogenase lipid molecule with a nitroimidazole group as a hydrophobic tail to confer hypoxia sensitivity and a tertiary amine as a hydrophilic head group, DSPE-PEG2000 (distearoylphosphatidylethanolamine-polyethylene glycol 2000) and cholesterol, and siRNA encapsulated by electrostatic interaction. Studies have shown that MLP can effectively improve the uptake efficiency of polo-like kinase 1 siRNA (siPLK1) in GBM cells due to increased positive charges due to low pH and hypoxia. Therefore, MLP/siPLK1 has been indicated to be a very effective siRNA delivery system by inhibiting the growth of GBM cells.23 Ojeda et al. developed ILs containing three ionizable glycerol lipids that showed good transfection efficiency in the rat's cerebral cortex. In HEK-293 cells (Human Embryonic Kidney 293 cells), the transfection efficiency of ILs-1 was the highest (about 21%), which of ILs-2 was about 13% and which of ILs-3 was about 20%. It must be emphasized that the transfection efficiency in cell culture of serine alcohol lipids previously modified with the same lipid tail is zero. These results offer new references for developing new non-viral carriers based on ILs and their application in the effective nucleic acid delivery to the brain.11
Recently, Tamaru et al. developed two liposome nanoparticles containing proton-ionizable amino lipids as carriers for encapsulating siRNA and pDNA in the same year. These Apolipoprotein E (Apo-E)-modified nanoparticles can be absorbed through endocytosis, avoiding lysosomal degradation and allowing for the nucleic acid delivery to brain endothelial cells.9, 24
3.3 Ionizable polymer-lipid nanoparticles (IPLNs)
The IPLNs are composed of a polymer core and lipid shell, which integrate the properties of LNPs and polymeric nanoparticles regarding physical stability and biocompatibility. When comparing with LNPs and liposomes, IPLNs show higher cell delivery efficiency.25 Mancini et al. prepared four phospholipid (APOE) liposome nano-formulations and plant-based ionizable polymer carriers. These nano-formulations were designed to promote the functionalization of peptide ligands for crossing the BBB and are multi-functional nano-vehicles that contain high levels of phenols, which are expected to treat depression. This plant-based complex formulation showed superior binding efficiency, enzyme inhibition, and scavenging activity and was able to improve the permeability of IL nano-formulations alone in cell monolayer studies.26
Dong Hee Kim et al. proposed a pH-sensitive carbamate sulfamethazine poly urethane amino sulfamethazine (PUASM) polymer micelle containing stromal cell-derived factor-1 alpha (SDF-1α) in their study. The targeted delivery of SDF-1α enhances angiogenesis in the CNS without affecting cell survival or inflammation. This polymer micelle can regulate the transmission of molecules in the microenvironment after a cerebral infarction, thereby improving the regeneration potential of brain tissue.27 Many IPLNs are largely dependent on electrostatic interactions between cationic biomaterials and anionic nucleic acids, leading to self-assembly into dynamic nanoscale ionizable complexes of polymers or lipids. The overexpression of GBM cells produces a peptide, Angiopep-2 (Ang), that targets low-density lipoprotein receptor-related protein (LRP), providing a dual targeting function for tissue-mediated delivery to the brain and cell targeting of GBM cells.28 Qiao et al. used a maleimide polymer (MP) with siRNA and a reactive oxygen species (ROS)-responsive zwitterionic lipid as a nanocarrier to deliver the targeted tumor growth factor β (siTGF-β) to obtain Ang-MP@siRNA IPLNs. In vitro experiments showed that the Ang-MP@siRNA IPLNs promoted the uptake of mouse glioma cells (GL261 cells) after passing through the cell monolayer simulating the BBB endothelium. In an in vivo study with an orthotopic glioma mouse model, its formulation enhanced the accumulation of siRNA in brain tumors.29
4 THE APPLICATION OF INs IN TREATING BRAIN DISEASES
Brain diseases, such as stroke, cerebral infarction, brain tumors, and Alzheimer's, are the leading cause of disability and death worldwide. The advent of gene therapy and the rapid advancement of genome engineering technology have opened up new avenues for the treatment of CNS diseases through the use of INs to encapsulate nucleic acids, such as siRNA, miRNA, and pDNA, or other synthetic drugs. This approach has the potential to effectively target brain tissue and treat CNS diseases.1
4.1 Delivery of nucleic acids by INs
The greatest advantage of INs is their ability to deliver a wide range of cargo as they are highly efficient at encapsulating and precisely releasing anionic drugs into cells.2 Some INs have also been found to show low immunogenicity and toxicity, highlighting their great delivery advantage to the brain by crossing the BBB. Therefore, they are particularly suitable for the delivery of all nucleic acids, including siRNA, mRNA, ASOs, and pDNA.
4.1.1 Small interfering RNA (siRNA)
siRNA is a short double-stranded RNA that targets and silences genes in a sequence-specific manner. Hypoxia-responsive ILs are developed to enhance the siRNA uptake in cells under hypoxic and low pH conditions, thereby providing a promising approach for treating glioma. The ILssiRNA system has the potential to overcome the challenges involved in delivering siRNA efficiently and effectively to the CNS. However, the safe and effective delivery of siRNA is critical to its success in targeting key signaling pathways and metabolism in brain cells. In this regard, cationic LNPs have shown promising results due to their biocompatible nature and high loading efficiency.
Studies have demonstrated the efficacy of LNP-siRNA delivery systems. For instance, Yathindranath et al. prepared an LNP for siRNA delivery (LNP-siSAT1) targeting spermidine/spermine N1-acetyltransferase 1 (SAT1) gene expression. The IN selectively sensitized potential gene targets in GBM cells and effectively reduced the SAT1 expression in brain-associated cells, such as human cerebral microvascular endothelium cells (hCMEC/D3), astrocytes, and macrophages, without causing significant cytotoxicity.30
In addition, Shuhan Liu et al. developed an ILNP that effectively delivers siRNA to the mouse brain by optimizing the chemical and physical properties of the ILNP. The regulation of the tumor microenvironment of GBM multiforme with the ILNP showed promising results and holds potential for the treatment of other brain tumors.31
4.1.2 Messenger RNA (mRNA)
The mRNA nucleic acid mechanism is a complex network that can monitor a wide range of biological processes.32 It is found that mRNA shows potential in gene therapy for treating and preventing a wide range of diseases by inducing protein production. While great progress has been made in designing mRNA with high efficiency, low innate immunogenicity, and low cost, the wide application of mRNA also requires effective and safe in vivo delivery technology. To this end, Guimaraes et al. designed a platform to accelerate mRNA delivery and screening, which consists of an ILNP library of barcoded miRNA (b-mRNA) with encapsulation functionality and custom design.33 Their experiments showed that the ILNP library with high levels of b-mRNA delivery in the context of brain tissue also had a high luciferase expression.
4.1.3 Plasmid DNA (pDNA)
pDNA is a double-stranded closed circular DNA that can replicate independently outside the chromosome or the nuclear region. It exists in a supercoiled state and is mainly found in bacteria, actinomycetes, and fungal cells. As gene vectors, plasmids have the capacity for autonomous replication and transcription and can maintain a constant copy number in progeny cells and express the genetic information carried. pDNA is widely used in gene therapy, cell therapy, and nucleic acid vaccines due to its safety, low toxicity, low immunogenicity, and easy separation and extraction. The ILS containing positively charged ionizable aminolipids multifunctional envelope-type nano device (YSK-MEND) is used to transport pDNA. The evaluation of fluorescently labeled APOE/YSK-MEND and fluorescent protein (mCherry) expression showed that this pDNA delivery system promotes transgene expression, indicating that it could be a promising tool for treating neurodegenerative diseases.24 Developing new non-viral carriers is crucial for finding more effective treatments for brain diseases. Ojeda et al. prepared nioplexes with different lipid/DNA ratios by adding the pCMS-EGFP plasmid to ionizable lipids. The evaluation of this ILNP in vivo showed good transfection efficiency after administration to the rat's cerebral cortex.11
In comparison with other nucleic acids, pDNA has a short history of development, their molecular structure is relatively large, and the loading rate of INs is low. Technical bottlenecks remain in the development and industrialization of their preparation processes. Additionally, as their active ingredient is pDNA, low transfection efficiency, low expression of target products, and unstable plasmid conformation pose great challenges to their development.
4.1.4 Antisense oligonucleotides (ASOs)
ASOs are single-stranded oligonucleotide molecules with a length of 12–30 bp. They generally comprise hybrid molecules of DNA and RNA with a low molecular weight. ASOs can bind to targets, such as mRNA or pre-mRNA, induce degradation, hinder translation, and shear capture to regulate expression. These functions can be applied to the design of ASO drugs for different targets, such as combining them with INs to form nanomedicines. For example, Dravet syndrome (DS) is a kind of intractable epileptic encephalopathy mainly caused by sodium channel, voltage-gated, type I, alpha (SCN1A) gene mutation, resulting in the haploid dysfunction of the voltage-gated sodium channel α subunit. SCN1A can be used as a target for the treatment of DS. Han et al. regulated the expression of SCN1A mutant gene by loading ASO into INs for targeted delivery to the mouse brain. Their experiments showed that sudden unexpected death in epilepsy (SUDEP) in the mouse model was significantly reduced.34 At present, eight ASO drugs have been marketed for the treatment of CNS, infection, tumors, and cardiovascular and other diseases.
The use of ASOs is a potential therapeutic strategy for the treating brain diseases. However, the transport of ASOs to the brain is challenging since ASOs cannot directly cross the BBB.35 Loading ASOs into INs can solve the problem of weak targeted delivery to the brain. Calero et al. integrated cell-permeable DNA-ASO into LNPs to interfere with mRNA expression. The prepared INs (ASO@LNP) can be targeted to brain macrophages and become a platform for the treatment of CNS diseases related to silent neuroinflammation.36 Yulian Wang et al. combined anti-TNF-α@ASO with cationic konjac glucomannan (cKGM) to form IPLNs. These INs have exceedingly high delivery efficiency to the brain. Further studies have shown that ASO-loaded IPLNs penetrate the BBB, are swallowed by BMECs, and are eventually absorbed by microglia in the brain and thus can be used to treat Parkinson's disease (PD).37
4.2 Brain diseases treated with INs
INs are used for the encapsulation of nucleic acids and drugs for the treatment of brain diseases, such as AD, PD, Huntington's disease (HD), brain tumors, stroke, depression, and multiple sclerosis (MS). AD is a complex disease, and there is still no effective anti-AD drug in the clinic. Grabowska-Pyrzewicz et al. proposed a multi-target treatment method based on mRNA, miRNA, and synthetic ASO drugs used for brain-targeted treatment.38
4.2.1 The microenvironment of brain disease and delivery strategy of NA by INs
As one of the most common types of brain tumors, glioma and its most aggressive form GBM, have gained limited progress in recent decades in part due to a lack of effective drug delivery strategies.39 An important factor in promoting the growth of GBM is the communication between tumor cells and other cells in the brain microenvironment, which promotes tumor cell growth and resistance to treatment. GBM recruits innate immune cells and changes their phenotype to accelerate tumor growth. Tumor cells can also change normal brain cells, such as endothelial cells, neurons, and astrocytes, forming a microenvironment conducive to tumor growth. The anionic lipids on the surface of endothelial cells and glioma cells suggest that selective drug delivery using cationic nanoparticles is a viable strategy. Cationic micelles can be engineered as carriers to selectively deliver compounds to glioma after intra-arterial injection.
Mancini et al. screened multi-effect medicinal plants with anti-depression activity and prepared liposome and plant compound nano-preparations with four phospholipids. These nano-preparations were used to enhance the functionalization of peptide ligands for BBB crossing, through which INs can help in the treatment of depression.26
4.2.2 Combination of other therapeutic modalities and IN gene therapy
IN gene therapy can be combined with other therapeutic modalities for brain diseases, including photothermal therapy (PTT), immunotherapy, and targeted stem cell therapy for brain cancer. S. Liu et al. prepared a new type of ILNP for brain tumor-targeting siRNA delivery and regulation of the tumor microenvironment during GBM immunotherapy. By designing the ILNP with different ionizable amine head groups, the authors identified lipids with the best acid dissociation constant (pKa; called BAMPA-O16B) and then combined them with siRNA to generate BAMPA-O16B/siRNA, which can significantly enhance the cellular uptake of siRNA in mouse GBM cells. Additionally, these ILNPs are very effective in downregulating the expression of target genes in tumors, thereby synergistically activating T cell-dependent anti-tumor immunity in GBM in situ.31 This is a typical combination of gene therapy and immunotherapy for the treatment of brain diseases.
PTT is a minimally invasive alternative for the treatment of drug-resistant CNS diseases. However, its poor permeability and potential off-target effects limit its clinical application. J.S. Liu et al. prepared and characterized polydopamine ionizable nanoparticles (PDA-ILNPs). These nanoparticles exhibit excellent photothermal conversion efficiency and can be used to ablate deep brain structures in vitro and in vivo in combination with near-infrared (NIR) light. PDA-ILNPs showed no significant cytotoxicity in the neuron-like SH-SY5Y cell line. However, when combined with NIR, they led to significant cell death, precise ablation of deep brain structures, and an ablation volume of about 6.5 mm3. These results demonstrate the potential of INs@PTT in the treatment of non-neoplastic CNS diseases.40
5 FUTURE PERSPECTIVES AND CHALLENGES
Nucleic acid drugs show significant advantages of clear treatment target, easy preparation, and lasting efficacy,7 making them ideal for treating brain diseases. However, biological barriers to the systemic nucleic acid delivery for brain diseases must be overcome through a precise vector design.
It is encouraging to see progress in brain drug delivery with INs as the “leaders” in effectively crossing the BBB. However, the INs used for delivery to the brain are not enough, and clinical transformation products still face great challenges at present. Existing nanocarriers are only applied in the treatment of a few neurological diseases, while other diseases, such as AD, are lacking in this regard.
Since active targeting is still lacking for the currently available INs, it is critical to incorporate brain-targeting modification strategies into ionizable nanomaterials. These strategies will allow the enhanced BBB uptake through active transporters and targeting ligands or receptors.2 Some endogenous ligands or monoclonal antibodies can act as molecular “Trojan horses” to bind exofacial epitopes on BBB receptor-mediated transport systems.3
Despite the rapid advancements in drug development in recent years, therapies for brain diseases still have a low success rate. Most of the research results revolve around INs@NA delivery systems sensitive to the microenvironment of brain tumors, the interaction between nanoparticles and brain tumors (including the general mechanism of interaction and targeted signaling pathways), and various treatment modalities for brain tumors (including PTT, gene therapy, and immunotherapy). There are few results on other brain diseases, such as stroke and MS. This is due to the complexity of the brain and its protected microenvironment, as well as the difficulty of translating preclinical findings into successful clinical candidates. The continued exploration of INs is likely to further promote progress in gene therapy for brain diseases.
In conclusion, there is scope for the further development of INs for nucleic acid delivery to the brain, and more researchers and companies are expected to focus on INs. Significant progress has been made in the application of INs for the treatment of other diseases, which provides references and potential strategies for the treatment of CNS diseases. Thus, more clinical applications are expected to be seen in the near future.
AUTHOR CONTRIBUTIONS
Ruru Xiong: Writing – original draft. Guixia Ling: Writing – review & editing. Yuqi Zhang: Resources. Jibin Guan: Writing – review & editing. Peng Zhang: Writing – review & editing.
ACKNOWLEDGMENTS
Not applicable.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




